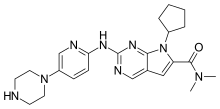
Ribociclib
 | |
| Clinical data | |
|---|---|
| Trade names | Kisqali |
| Other names | LEE 011 |
| AHFS/Drugs.com | kisqali |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ~70% |
| Metabolism | Liver (CYP3A4) |
| Elimination half-life | 32.0 (29.7–54.7) hrs |
| Excretion | 69% feces, 32% urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.234.566 |
| Chemical and physical data | |
| Formula | C23H30N8O |
| Molar mass | 434.548 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ribociclib (trade name Kisqali[1]) is an inhibitor of cyclin D1/CDK4 and CDK6, and is used for the treatment of certain kinds of breast cancer.[2] It is also being studied as a treatment for other drug-resistant cancers.[3] It was developed by Novartis and Astex Pharmaceuticals.[4]
Medical uses
Ribociclib was approved by the US FDA in March 2017 and the European Medicines Agency in August 2017 for use in combination with an aromatase inhibitor to treat HR-positive, HER2-negative advanced or metastatic breast cancers.[1][5]
Interactions
As ribociclib is mainly metabolized by the liver enzyme CYP3A4, inhibitors of this enzyme increase its concentrations in the body and could potentiate side effects and toxicity. Examples of such inhibitors include ketoconazole and similar antifungal drugs, ritonavir, clarithromycin, as well as grapefruit. Conversely, drugs that induce CYP3A4, such as rifampicin and St John's Wort, can decrease ribociclib concentrations.[2][5]
Ribociclib itself is a moderate to strong CYP3A4 inhibitor and therefore can increase concentrations of other drugs that share this metabolism, as has been shown with midazolam. It also inhibits a number of transporter proteins and could thus theoretically interfere with the transport of other drugs. It could also amplify QT prolongation of other drugs such as antiarrhythmics, clarithromycin, and haloperidol.[2][5]
Pharmacology
Mechanism of action
Cyclin-dependent kinases (CDKs) 4 and 6 are enzymes that have been shown to promote cell division and multiplication in both normal and cancer cells. Many cancer cells have shown abnormalities that increase the activity of CDK, leading to the inactivation of certain tumor suppressor genes.[3][6] This has led to the idea that inhibiting CDK4 will slow the growth of tumors by reactivating these tumor suppressors.
When used in combination with other drugs such as an ALK or an MEK inhibitor, ribociclib has been shown to have a synergistic effect, resulting in improved responses.[7][8] Again, this is likely a result of "crosstalk" between signaling pathways. Simply blocking one pathway in cancer tumorigenesis can sometimes result in "tumor compensation", where the tumor compensates for the blocked signaling pathway by utilizing other pathways to survive. By blocking several pathways at once, it is thought that the tumor is less able to compensate, and a greater anti-tumor response is often observed. It is also of interest that utilizing ribociclib in combination with other agents has been shown to reduce the development of resistance to these agents.[3] In other words, cancer’s development of drug resistance can be mitigated with the addition of ribociclib to the therapeutic regime.
Clinical trials
Ribociclib has been shown to be well-tolerated, although its best response as a single agent as of 2014 has been stable disease.[9] In October 2016, good results (increased progression-free survival) were reported from the MONALEESA-2 trial (in combination with letrozole) in metastatic breast cancer.[10]
As of September 2017[update], ribociclib is in phase II development for several indications, including liposarcoma,[11] endometrial carcinoma[12] and neuroendocrine tumors of the foregut.[13]
See also
- Palbociclib, similar mechanism and indications
References
- ^ a b FDA Clears Novartis Kisqali for Combination Breast Cancer Therapy. March 2017
- ^ a b c FDA Professional Drug Information on Kisqali. Accessed 2017-09-08.
- ^ a b c Samson, Kurt (2014). "LEE011 CDK Inhibitor Showing Early Promise in Drug-Resistant Cancers". Oncology Times. 36 (3): 39–40. doi:10.1097/01.COT.0000444043.33304.c1.
- ^ "Novartis LEE011 (ribociclib) granted FDA Priority Review for first-line treatment of HR+/HER2- advanced breast cancer". Novartis. 2016-11-01.
- ^ a b c "Kisqali: EPAR – Product Information" (PDF). European Medicines Agency. 2017-08-31.
- ^ Kim, S.; Loo, A.; Chopra, R.; Caponigro, G.; Huang, A.; Vora, S.; Parasuraman, S.; Howard, S.; Keen, N.; Sellers, W.; Brain, C. (2014). "Abstract PR02: LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6- Reactivating Rb in cancer". Molecular Cancer Therapeutics. 12 (11_Supplement): PR02. doi:10.1158/1535-7163.TARG-13-PR02.
- ^ Sosman, Jeffrey Alan; Kittaneh, Muaiad; Lolkema, Martijn P. J. K.; Postow, Michael Andrew; Schwartz, Gary; Franklin, Catherine; Matano, Alessandro; Bhansali, Suraj; Parasuraman, Sudha; Kim, Kevin (2014). "A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity". Journal of Clinical Oncology. 32 (15 Suppl): 9009.
- ^ Wood, Andrew C.; Krytska, Kateryna; Ryles, Hannah; Sano, Renata; Li, Nanxin; King, Frederick; Smith, Timothy; Tuntland, Tove; Kim, Sunkyu; Caponigro, Giordano; He, You Qun; Jennifer, Harris; Mosse, Yael (2014). "Abstract 1000: Combination CDK4/6 and ALK inhibition demonstrates on-target synergy against neuroblastoma". Cancer Research. 74 (19 Supplement): 1000. doi:10.1158/1538-7445.AM2014-1000.
- ^ Geoerger, B.; Bourdeaut, F.; Dubois, S.G.; Dewire, M.D.; Marabelle, A.; Pearson, A.D.; Modak, S.; Kan, R.; Matano, A.; Bhansali, S.G.; Parasuraman, S.; Chi, S.N. (2014). "455P Phase I study of LEE011 (CDK4/6 inhibitor) in patients with malignant rhabdoid tumors, neuroblastoma, and cyclin D–CDK4/6 pathway-activated tumors". Annals of Oncology. 25 (suppl 4): iv151–2. doi:10.1093/annonc/mdu331.15.
- ^ Anti-CDK4/6 Boosts PFS in Metastatic Breast Cancer. Oct 2016
- ^ Clinical trial number NCT03096912 for "A Study Assessing Efficacy & Safety of Ribociclib in Patients With Advanced Well/Dedifferentiated Liposarcoma" at ClinicalTrials.gov
- ^ Clinical trial number NCT03008408 for "Study of Ribociclib (LEE011), Everolimus, and Letrozole, in Patients With Advanced or Recurrent Endometrial Carcinoma" at ClinicalTrials.gov
- ^ Clinical trial number NCT02420691 for "LEE011 in Neuroendocrine Tumors of Foregut Origin" at ClinicalTrials.gov
