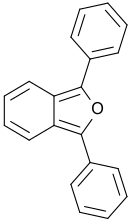
1,3-diphenylisobenzofuran
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,3-diphenylisobenzofuran | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 20 H 14 O | |||||||||||||||
| Brief description |
pale yellow to dark yellow crystalline powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 270.33 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
practically insoluble in water, soluble in many solvents, such as. B. acetonitrile , benzene , dichloromethane , chloroform , dimethyl sulfoxide , tetrahydrofuran and toluene |
|||||||||||||||
| Refractive index |
1.6700 at 25 ° C (589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,3-Diphenylisobenzofuran is a highly reactive diene that can also trap unstable and short-lived dienophiles in a Diels-Alder reaction and is used as a standard reagent for the determination of singlet oxygen - also in biological systems.
Cycloadditions with 1,3-diphenylisobenzofuran with subsequent elimination of oxygen open up access to a large number of polyaromatics .
Occurrence and representation
The first synthesis of 1,3-diphenylisobenzofuran was reported in 1905 by A. Guyot and J. Catel. The reaction of phenylmagnesium bromide with 3-phenylphthalide (e.g. from the methyl ester of 3-hydroxyphthalide with phenylboronic acid in 95% yield) results in a lactol which, with mineral acids , gives 1,3-diphenylisobenzofuran in 87% yield with dehydration.
In the patent literature, the preparation of 1,3-diphenylisobenzofuran by [4 + 2] cycloaddition of 1,3-butadiene and dibenzoylethylene (1,4-diphenyl-2-buten-1,4-dione, from fumaroyl chloride and benzene in Presence of aluminum chloride ). The dibenzoylethylene, which is predominantly in the trans configuration, is converted into the cis configuration required for addition when heated .
The 4,5-dibenzoylcyclohexene formed is cyclized with acetic anhydride to give dihydroisobenzofuran, which in turn is opened to 1,2-dibenzoylbenzene by addition of bromine and hydrogen bromide elimination and is cyclized with zinc - acetic acid to give the end product 1,3-diphenylisobenzofuran. A publication from 1940 describes high yields at the individual stages of the long reaction sequence.
Phthaloyl chloride is a much cheaper starting material for 1,2-dibenzoylbenzene by Friedel-Crafts acylation with benzene, which is reduced to 1,3-DPBF in 78% yield by potassium borohydride .
1,3-Diarylisobenzofurans from 2-acylbenzaldehydes and boronic acids can be used less laboriously and with better yields
or from salicylaldehydes via phenacylhydrazones, the oxidation of which with lead (IV) acetate to ortho -diketones and subsequent reaction with an aryl Grignard compound are obtained.
properties
1,3-Diphenylisobenzofuran is a yellow, light- and air-sensitive, crystalline and soluble in many organic solvents with a maximum absorption in solution around 420 nm, which produces an intense fluorescence. Because of the stability of 1,3-DPBF in DMF and DMSO fluorescence measurements can be carried out in these solvents, while the dissolved isobenzofuran even in the absence of oxygen in CHCl 3 and CCl 4 quickly by attack of CHCl 2 - or CCl 3 - radicals photolyzed becomes.
1,3-Diphenylisobenzofuran forms an orange-yellow, fluorescent solution in ethanol which, when irradiated in the absence of oxygen, turns into a colorless, non-fluorescent solution through the formation of a colorless crystal-forming photodimer.
use
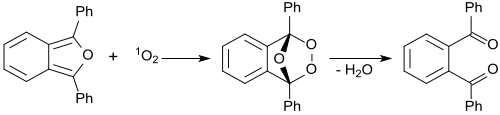
Reagent for the determination of singlet oxygen
In the presence of methylene blue irradiated with red laser light , 1,3-diphenylisobenzofuran reacts with singlet oxygen 1 O 2 formed as an intermediate to form an unstable peroxide, which breaks down into (colorless) 1,2-dibenzoylbenzene.
The detection of singlet oxygen by 1,3-diphenylisobenzofuran is based on this reaction, also in biological systems. For this purpose, water-soluble derivatives of 1,3-DPBF have also been developed as 1 O 2 scavengers.
Dienophile in Diels-Alder reactions
Isobenzofurans, such as 1,3-diphenylisobenzofuran, are among the most reactive Diels-Alder dienes currently known and are suitable for trapping short-lived and unstable olefins and alkynes. The working group around Georg Wittig made important contributions to this.
1,3-DPBF reacts with the less stable cyclohexine to form a tricyclic adduct which, after hydrogenation and elimination of hydrogen, forms a 9,10-diphenylcyclohexenonaphthalene.
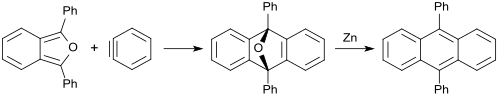
With aryne (dehydrobenzene), an oxygen-bridged anthracene is produced analogously in 85% yield, which can be reduced with zinc to 9,10-diphenylanthracene (88% yield).
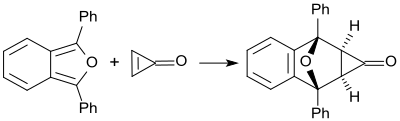
The cyclopropenone , which is unstable at temperatures above its melting point (−29 ° C), reacts quantitatively with 1,3-diphenylisobenzofuran at room temperature to form a Diels-Alder adduct,) which is exclusively present as an exo isomer.
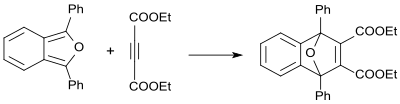
Dimethyl acetylenedicarboxylate as a dienophile reacts with 1,3-diphenylisobenzofuran in 84% yield to form the corresponding adduct.
Also with heterocyclic dienophiles, such as. B. 3-sulfolene forms 1,3-diphenylisobenzofuran the corresponding Diels-Alder adduct.
Molecular building block for the construction of polyaromatics
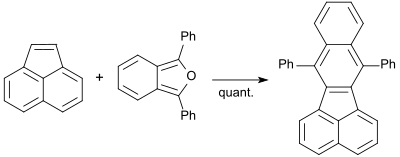
Polyaromatic hydrocarbons (PAH) are of great interest in their properties as precursors for graphite , but especially as environmental pollutants with pronounced persistence and carcinogenicity . 1,3-DPBF also reacts quantitatively with acenaphthylene when heated to 160 ° C to form 7,12-diphenylbenzo [ k ] fluoranthene.
The twofold Diels-Alder reaction of 1,3-DPBF with p-benzoquinone gives practically quantitatively an adduct which with p-toluenesulfonic acid gives a pentacene derivative in 49% yield .
literature
- W. Friedrichsen: Benzo [c] furans . In: Adv. Heterocycl. Chem. Band 26 , 1980, pp. 135-234 , doi : 10.1016 / S0065-2725 (08) 60141-5 .
- W. Friedrichsen: Recent advances in the chemistry of benzo [c] furans and related compounds . In: Adv. Heterocycl. Chem. Band 73 , 1999, pp. 1-96 , doi : 10.1016 / S0065-2725 (08) 600940-X (English).
- R. Rodrigo: Progress in the chemistry of isobenzofurans: Applications to the synthesis of natural products and polyaromatic hydrocarbons . In: Tetrahedron . tape 44 , no. 8 , 1988, pp. 2093-2135 , doi : 10.1016 / S0040-4020 (01) 81720-8 (English).
Individual evidence
- ↑ Entry on 1,3-diphenylisobenzofuran at TCI Europe, accessed on July 27, 2017.
- ↑ a b c d data sheet 1,3-diphenylisobenzofuran 97% from Sigma-Aldrich , accessed on July 27, 2017 ( PDF ).
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 604 .
- ^ A b R. Adams, MH Gold: The Synthesis of 1,3-Diphenyldihydroisobenzofurans, 1,3-Diphenylisobenzofurans and o-Dibenzoylbenzenes from the Diene Addition Products to Dibenzoylethylene . In: J. Am. Chem. Soc. tape 62 , no. 1 , 1940, p. 56-61 , doi : 10.1021 / ja01858a012 .
- ↑ PC Kierkus: 1,3-Diphenylisobenzofuran . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rd420 .
- ↑ RH Young, K. Wehrly, RL Martin: Solvent effects in dye-sensitized photooxidation reactions . In: J. Am. Chem. Soc. tape 93 , no. 22 , 1971, p. 5774-5779 , doi : 10.1021 / ja00751a031 .
- ↑ JA Howard, GD Mendenhall: Autoxidation and photooxidation of 1,3-diphenylisobenzofuran: A kinetic and product study . In: Can. J. Chem. Volume 53 , no. 14 , 1975, p. 2199-2201 , doi : 10.1139 / v75-307 .
- ↑ P. Carloni et al .: On the use of 1,3-diphenylisobenzofuran (DPBF). Reactions with carbon and oxygen-centered radicals in model and natural systems . In: Res. Chem. Intermed. tape 19 , no. 5 , 1993, p. 395-405 , doi : 10.1163 / 156856793X00181 .
- ↑ A. Guyot, J. Catel, Compt. Rend. Hebd. Acad. Sci., Ser. C140, 1348 (1905)
- ↑ A. Guyot, J. Catel, Bull. Soc. Chim. France, [3] (35), 1124 (1906)
- ↑ M. Kuriyama, N. Ishiyama, R. Shimazawa, R. Shirai, O. Onomura: Efficient synthesis of 3-arylphthalides using palladium-catalyzed arylation of aldehydes with organoboronic acids . In: J. Org. Chem. Band 74 , no. 23 , 2009, p. 9210-9213 , doi : 10.1021 / jo901964k .
- ↑ MS Newman: Evidence favoring a two-step mechanism for the Diels-Alder reaction . In: J. Org. Chem. Band 26 , no. 8 , 1961, pp. 2630-2633 , doi : 10.1021 / jo01066a004 .
- ^ RE Lutz: trans-Dibenzoylethylene In: Organic Syntheses . 20, 1940, p. 29, doi : 10.15227 / orgsyn.020.0029 ; Coll. Vol. 3, 1955, p. 248 ( PDF ).
- ↑ Patent US2325727 : Dehydroisobenzofurans and process for preparing them. Applied July 27, 1940 , published August 3, 1943 , applicant: EI du Pont de Nemours & Co., inventor: R. Adams.
- ↑ Data sheet 1,2-Dibenzoylethylene, predominantly trans, 96% from AlfaAesar, accessed on August 22, 2017 ( PDF )(JavaScript required) .
- ^ DV Klemm, A. Tuncay: Photochemical and thermal isomerization of trans- and cis-1,2-dibenzoylethylene: A microscale approach . In: J. Chem. Educ. tape 66 , no. 6 , 1989, pp. 519 , doi : 10.1021 / ed066p519 .
- ↑ Patent US2356907 : 1,3-Diphenylisobenzofurans and process for preparing the same. Applied July 27, 1940 , published August 29, 1944 , applicant: EI du Pont de Nemours & Co., inventor: R. Adams.
- ↑ Houben-Weyl Methods of Organic Chemistry, Vol. XIII / 2a: Organometallic Compounds of Group II of the Periodic Table, 4th Edition . Thieme, Stuttgart 1973, ISBN 978-3-13-213204-7 , p. 419 .
- ↑ M. Cava, MJ Mitchell, AA Deana: Condensed cyclobutane aromatic compounds. XIII. An attempted synthesis of 1,2-diphenylbenzocyclobutene . In: J. Org. Chem. Band 25 , no. 9 , 1960, pp. 1481-1484 , doi : 10.1021 / jo01079a005 .
- ↑ J. Jacq, B. Bessières, C. Einhorn, J. Einhorn: Regiospecific synthesis of functionalized 1,3-diarylisobenzofurans via palladium- and rhodium-catalysed reaction of boronic acids with o-acylbenzaldehydes under thermal or microwave activation . In: Org. Biomol. Chem. Band 8 , 2010, p. 4927-4933 , doi : 10.1039 / 0OB00110D .
- ↑ A. Kotali, PG Tsoungas: Oxidation of N-aroylhydrazones of o-hydroxyaryl ketones with lead (IV) acetate: A facile route to aromatic o-diketones . In: Tetrahedron Lett. tape 28 , no. 37 , 1987, pp. 4321-4322 , doi : 10.1016 / S0040-4039 (00) 96497-9 .
- ↑ J. Jacq, C. Einhorn, J. Einhorn: A versatile and regiospecific synthesis of functionalized 1,3-diarylisobenzofurans . In: Org. Lett. tape 10 , no. 17 , 2008, p. 3757-3760 , doi : 10.1021 / ol80155a .
- ↑ M. Wozniak, F. Tanfani, E. Bertoli, G. Zolese, J. Antonsiewicz: A new fluorescence method to detect singlet oxygen inside phospholipid model membranes . In: Biochim. Biophys. Acta . tape 1082 , no. 1 , 1991, p. 94-100 , doi : 10.1016 / 0005-2760 (91) 90304-Z .
- ↑ X.-F. Zhang, X. Liu: The photostability and fluorescence properties of diphenylisobenzofuran . In: J. Luminiscence . tape 131 , no. 11 , 2011, p. 2263-2266 , doi : 10.1016 / j.jlumin.2011.05.048 .
- ^ A. Schoenberg, A. Mustafa, G. Aziz: Diels-Alder Reaction. II. Experiments with 2-styrylchromones. On the Nature of the Dimer of 1,3-Diphenylisobenzofuran . In: J. Am. Chem. Soc. tape 76 , no. 18 , 1954, p. 4576–457722 , doi : 10.1021 / ja01647a020 .
- ↑ Basic practical course in physical chemistry, V28, photooxidation of diphenylisobenzofuran, investigation of reaction kinetics by photometry. (PDF) Ulm University, December 6, 2010, accessed on August 30, 2017 .
- ↑ C. Schmitz, JM Aubry, J. Rigaudy: A new access to the anthracene core: Synthesis of two water soluble singlet oxygen traps derived from 1,3-diphenylisobenzofuran and 9,10-diphenylanthracene . In: Tetrahedron . tape 38 , no. 10 , 1982, pp. 1425-1430 , doi : 10.1016 / 0040-4020 (82) 80224-X .
- ↑ D. Tobia, B. Rickborn: Substituent effects on rates of inter- and intramolecular cycloaddition reactions of isobenzofurans . In: J. Org. Chem. Band 52 , no. 12 , 1987, pp. 2611-2615 , doi : 10.1021 / jo00388a055 .
- ↑ G. Wittig: About small rings with carbon triple bonds - another chemistry of "As if" . In: Pure Appl. Chem. Band 7 , no. 2-3 , 1963, pp. 173-192 , doi : 10.1351 / pac196307020173 .
- ↑ G. Wittig, E. Knauss, K. Niethamer: About 9,10-dihydroanthracene derivatives with hetero bridging atoms . In: Justus Liebigs Ann. Chem. Band 630 , no. 1 , 1960, p. 10-18 , doi : 10.1002 / jlac.19606300103 .
- ^ R. Breslow, M. Oda: Isolation and characterization of pure cyclopropenone . In: J. Am. Chem. Soc. tape 94 , no. 13 , 1972, p. 4787-4788 , doi : 10.1021 / ja00768a089 .
- ↑ Exo selectivity of the Diels-Alder addition of cyclopropenone and 1-3-diphenylisobenzofuran. Retrieved August 28, 2017 .
- ↑ JA Berson: Reactions of 1,3-diphenylisobenzofuran with acetylenic dienophiles . In: J. Am. Chem. Soc. tape 75 , no. 5 , 1953, pp. 1240–1241 , doi : 10.1021 / ja01101a503 .
- ↑ MP Cava, JP VanMeter: Condensed cyclobutane aromatic compounds. XXX. Synthesis of some unusual 2,3-naphthoquinonoid heterocycles. A synthetic route to derivatives of naphtho [2,3-b] biphenylene and anthra [b] cyclobutene . In: J. Org. Chem. Band 34 , no. 3 , 1969, p. 538-545 , doi : 10.1021 / jo01255a012 .
- ↑ Houben-Weyl Science of Synthesis, Vol. 45b: Compounds with All-Carbon Functions . Thieme, Stuttgart 2009, ISBN 978-3-13-146551-1 , p. 1038 .
- ↑ GP Miller, J. Briggs: Progress towards the synthesis of tris and tetrakis [60] fullerene adducts . In: Electrochem. Soc. Proc. tape 2002-12 , 2002, ISBN 1-56677-333-4 , pp. 279-284 .