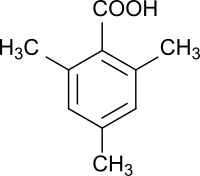
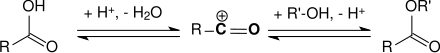2,4,6-trimethylbenzoic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,4,6-trimethylbenzoic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 12 O 2 | |||||||||||||||
| Brief description |
white to beige solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 164.20 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point | ||||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,4,6-Trimethylbenzoic acid is an aromatic carboxylic acid substituted with three methyl groups , the carboxy group of which is sterically hindered by two methyl groups in the ortho position .
presentation
A synthesis of 2,4,6-trimethylbenzoic acid starts from bromomesitylene , which is reacted in the presence of 2 molar ethyl bromide with magnesium turnings in diethyl ether to give the corresponding Grignard compound . The ether phase is poured onto dry ice , the magnesium bromide salt of 2,4,6-trimethylbenzoic acid being formed in a Grignard reaction . Subsequent acidification leads to the precipitation of the carboxylic acid, which is taken up in sodium hydroxide solution for purification and precipitated by renewed acidification.
2,4,6-Trimethylbenzoic acid is obtained in 86-87% yield, and recrystallization from 45% methanol gives the aromatic carboxylic acid in long needles in 84-86% yield.
The conventional variant of the Grignard reaction with iodine to start the reaction provides only 55-61% pure 2,4,6-trimethylbenzoic acid.
The reaction of mesitylene (from acetone and sulfuric acid ) with oxalyl chloride in carbon disulfide at 10-15 ° C and then at 40 ° C in the presence of aluminum chloride gives mesitylene carboxylic acid in yields of 65-76%.
Phenylglyoxylic acid chloride is initially formed, which is easily decarbonylated to mesitylene carboxylic acid chloride and then hydrolyzed to carboxylic acid with aqueous hydrochloric acid. The handling of CS 2 and of molar amounts of anhydrous AlCl 3 , as well as the development of hydrogen chloride and in particular of carbon monoxide make this reaction path unusable for industrial synthesis.
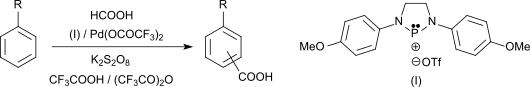
The Pd (II) -catalyzed carboxylation of aromatics with formic acid in the presence of phosphenium salts leads to aromatic carboxylic acids, including 2,4,6-trimethylbenzoic acid, in yields of up to 93%.
Mesitylene is also accessible to a Friedel-Crafts-like electrophilic aromatic substitution with carbon dioxide in the presence of silylium borates and gives 2,4,6-trimethylbenzoic acid in 66% yield.
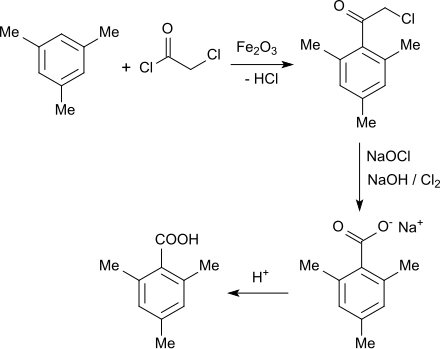
A two-stage technical process leads from mesitylene by Friedel-Crafts acylation with chloroacetic acid chloride in the presence of catalytic amounts of iron (III) oxide to α-chloro-2,4,6-trimethylacetophenone.
In a unicorn reaction , the chloromethyl ketone obtained is treated with alkaline sodium hypochlorite solution and other alkali metal carbonate solution and a phase transfer catalyst , e.g. B. Dimethyldibenzylammonium chloride added and heated to reflux after introducing chlorine . After isolation, purification and drying, 2,4,6-trimethylbenzoic acid is obtained in 90% yield.
properties
2,4,6-Trimethylbenzoic acid is a white, odorless solid that crystallizes in long needles, is sparingly soluble in water and readily soluble in alcohols.
Because of the steric hindrance of the carboxy group by the two methyl groups in the ortho position, 2,4,6-trimethylbenzoic acid cannot be esterified in the conventional way with acid catalysis. The carboxylic acid dissolves in 96% concentrated sulfuric acid with the formation of an acylium ion , which reacts in a two-stage and trimolecular equilibrium reaction through nucleophilic attack by the alcohol to form the ester.
use
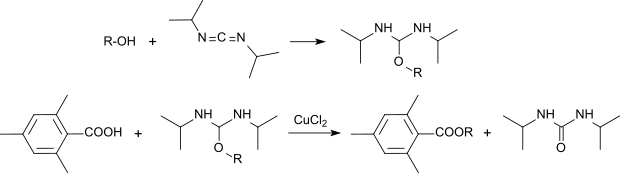
Esters of 2,4,6-trimethylbenzoic acid can be prepared by reacting with the O, N, N'-trisubstituted isourea formed from the peptide reagent diisopropylcarbodiimide and an alcohol in the presence of copper (II) chloride .
The ethyl ester of mesitylene carboxylic acid can also be obtained in 80% yield by reaction with ethyl iodide in the presence of equimolar amounts of DBU .
Because of its sterically demanding structure and its high stability against base hydrolysis, the mesitoyl group is used as a protective group for hydroxyl groups in natural product chemistry. The mixed anhydride of 2,4,6-trimethylbenzoic acid with trifluoroacetic acid is suitable for introducing the mesitoyl protective group . The esters obtained can be cleaved gently with lithium aluminum hydride LiAlH 4 or aluminum hydride AlH 3 .
Alkyl esters of mesitylene carboxylic acid can also be hydrolyzed in high yields (methyl ester: 93%, n-octyl ester: 87%) by a mixture of powdered potassium hydroxide and the phase transfer catalyst Aliquat 336 (methyltrioctylammonium chloride).
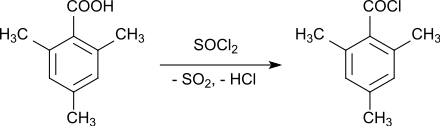
The mesitylene carboxylic acid with thionyl chloride in 90-97% yield
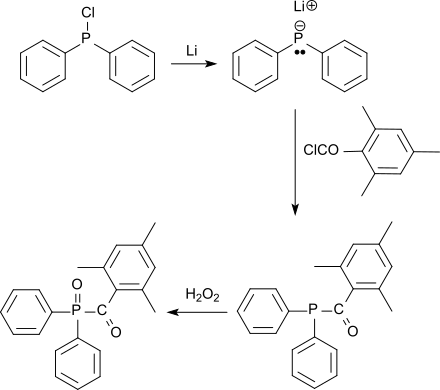
is an important starting compound for the production of so-called UV initiators of the acylphosphine oxide type, which decompose into radicals when exposed to visible or UV light and trigger chain polymerizations . For this UV radiation curing (English. UV curing ) u. a. the so-called mono-acylphosphine oxides (MAPO), such as. B. Trimethylbenzoyldiphenylphosphine oxide TPO are used, which are obtained from chlorodiphenylphosphine after lithiation and reaction with molar amounts of mesitoyl chloride to 2,4,6-trimethylbenzoyl-diphenylphosphine and subsequent oxidation with hydrogen peroxide to phosphine oxide TPO
As a representative of the so-called bis-acylphosphine oxides (BAPO), bis (2,4,6-trimethylbenzoyl) phenylphosphine oxide can be obtained from dichlorophenylphosphine after lithiation and reaction with two molar amounts of mesitoyl chloride to bis-2,4,6-trimethylbenzoyl-phenylphosphine and subsequent oxidation with hydrogen peroxide to phosphine oxide are obtained, which are characterized by low volatility and low odor.
Irradiation with two UV photons generates four polymerization-active radicals from a photoinitiator molecule of the BAPO type. Bis-acylphosphine oxides are therefore highly effective free- radical initiators , which are also suitable for through-cure of thick and highly pigmented coating systems.
See also
Individual evidence
- ↑ a b c data sheet 2,4,6-trimethylbenzoic acid for synthesis at Sigma-Aldrich , accessed on April 10, 2016 ( PDF ).
- ↑ a b c G.S. Hamilton: 2,4,6-trimethylbenzoic acid . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rt270 .
- ↑ DM Bowen: Mesitoic Acid, [β-Isodurylic acid], [I. METHOD A] In: Organic Syntheses . 21, 1941, p. 77, doi : 10.15227 / orgsyn.021.0077 ; Coll. Vol. 3, 1955, p. 553 ( PDF ).
- ↑ a b R.P. Barnes: [II. METHOD B], [Mesitoic Acid and Mesitoyl Chloride] In: Organic Syntheses . 21, 1941, p. 77, doi : 10.15227 / orgsyn.021.0077 ; Coll. Vol. 3, 1955, p. 553 ( PDF ).
- ↑ R. Adams, RW Hufferd: mesitylenes In: Organic Synthesis . 2, 1922, p. 41, doi : 10.15227 / orgsyn.002.0041 ; Coll. Vol. 1, 1941, p. 341 ( PDF ).
- ↑ PE Sokol: Mesitoic Acid In: Organic Syntheses . 44, 1964, p. 69, doi : 10.15227 / orgsyn.044.0069 ; Coll. Vol. 5, 1973, p. 706 ( PDF ).
- ↑ K. Sakakibara, M. Yamashita, K. Nozaki: An efficient Pd (II) -based catalyst system for carboxylation of aromatic C-H bond by addition of a phosphenium salt . In: Tetrahedron Lett. tape 46 , no. 6 , 2005, p. 959-962 , doi : 10.1016 / j.tetlet.2004.12.027 .
- ↑ M. Konno, M. Chiba, K. Nemoto, T. Hattori: Electrophilic aromatic substitution of arenes with CO2 mediated by R 3 SiB (C 6 F 5 ) 4 . In: Chem. Lett. tape 41 , no. 9 , 2012, p. 913-914 , doi : 10.1246 / cl.2012.913 .
- ↑ Patent US5296636 : Preparation of 2,4,6-trimethylenzoic acid. Applied on January 28, 1993 , published on March 22, 1994 , applicant: BASF AG, inventor: W. Siegel, R. Kropp, J. Schroeder.
- ↑ H. Shi, Y. Wang, R. Hua: Acid-catalyzed carboxylic acid esterification and ester hydrolysis mechanism: acylium ion as a sharing active intermediate via a spontaneous trimolecular reaction based on density functional theory calculation and supported by electrospray ionization-mass spectrometry . In: Phys. Chem. Chem. Phys. tape 41 , no. 9 , 2012, p. 913-914 , doi : 10.1246 / cl.2012.913 .
- ^ E. Däbritz: Syntheses and Reactions of O, N, N′-Trisubstituted Isoureas . In: Angew. Chem. Band 5 , no. 5 , 1966, pp. 470-477 , doi : 10.1002 / anie.196604701 .
- ↑ J. Otero, J. Nishikido: Esterification: Methods, Reactions, and Applications, 2nd Edition . Wiley-VCH, Weinheim 2010, ISBN 978-3-527-32289-3 , pp. 182 .
- ↑ S. Cai, S. Hakomori, T. Toyokuni: Application of protease-catalyzed regioselective esterification in synthesis of 6'-deoxy-6'-fluoro- and 6-deoxy-6-fluorolactosides . In: J. Org. Chem. Band 57 , no. 12 , 1992, pp. 3431-3437 , doi : 10.1021 / jo00038a036 .
- ↑ A. Loupy, M. Pedoussant, J. Sansoulet: Solid-liquid phase-transfer catalysis without solvent: mild and efficient conditions for saponifications and preparations of hindered esters . In: J. Org. Chem. Band 51 , no. 5 , 1986, pp. 740-745 , doi : 10.1021 / jo00355a032 .
- ↑ a b Patent WO2000032612 : Process for preparing acylphosphines and derivatives. Applied on November 20, 1999 , published on June 8, 2000 , Applicant: Ciba Specialty Chemicals Holding Inc., Inventors: D. Leppard, E. Eichenberger, R. Kaeser, G. Hug, U. Schwendimann.
- ↑ P. Glöckner, T. Jung, S. Struck, K. Studer: Radiation Curing for Coatings and Printing Inks: Technical Basics and Applications . Vincentz Network, Hannover 2008, ISBN 978-3-86630-907-4 , p. 37-39 .