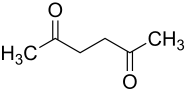
2,5-hexanedione
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,5-hexanedione | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 10 O 2 | ||||||||||||||||||
| Brief description |
clear, colorless, aromatic-smelling liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 114.14 g · mol -1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.974 g cm −3 |
||||||||||||||||||
| Melting point |
−5.4 ° C |
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| Vapor pressure |
0.6 mbar (20 ° C) |
||||||||||||||||||
| solubility |
easy in water (≥ 100 g l −1 at 22 ° C) |
||||||||||||||||||
| Refractive index |
1.423 (20 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
2,5-Hexanedione ( molecular formula C 6 H 10 O 2 ) is a diketone and a metabolite of n -hexane which is toxic for the human body .
Extraction and presentation
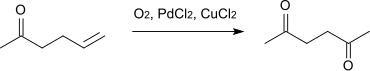
2,5-Hexanedione can be prepared by alkaline hydrolysis of diacetyl succinic acid ester and subsequent heating (ketone cleavage). It can also be made by oxidation of allylacetone .
The synthesis is also possible by oxidation of 2,5-dimethylfuran .
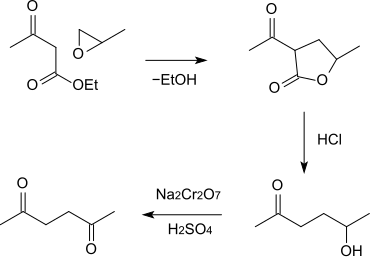
Another synthetic route, starting from acetoacetic ester and propylene oxide , has also been described. The condensation product, α-aceto-γ-valerolactone, is reacted with dilute hydrochloric acid to give 5-hydroxy-2-hexanone (acidic saponification and subsequent decarboxylation ), which is finally oxidized to 2,5-hexanedione with sodium dichromate and sulfuric acid .
properties
2,5-Hexanedione reacts, among other things, with the functional group of amines , such as those found in proteins . There it causes a crosslinking of the molecules and a loss of protein functionality. Crosslinking with the α - amino acid lysine leads to the formation of the aromatic 2,5-dimethyl pyrrole .
use
2,5-hexanedione is used as a solvent for cellulose acetate, tanning agents, varnishes and paints. It is also used as a protective group for primary amines and as an intermediate for organic synthesis and for the manufacture of pharmaceuticals.
Biological importance
The metabolite 2,5-hexanedione is an intermediate product in the course of the conversion of n -hexane to 3-methylcyclopent-2-en-1-one by intramolecular aldol condensation .
safety instructions
The substance can cause irritation of the eyes, skin and mucous membranes, degreasing and orange-brown skin inflammation and unconsciousness in humans. In the human body, 2,5-hexanedione occurs as a metabolite of hexane and is excreted in the urine. 2,5-hexanedione is much more toxic than n -hexane itself and leads to irreparable nerve damage due to the cross-linking reactions.
2,5-Hexanedione also has a toxic effect on spermatogenesis by damaging the Sertoli cells , which form the epithelium of the seminiferous tubules in the seminiferous tubules .
Individual evidence
- ↑ a b c Entry on 2,5-hexanedione. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- ↑ a b c d e Entry on 2,5-hexanedione in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c d e f data sheet 2,5-hexanedione (PDF) from Merck , accessed on May 16, 2011.
- ↑ a b c R. H. Lasco: Oxidation of allylacetone to 2,5-hexanedione in a water-carbon tetrachloride solvent system , US Patent 3972942.
- ^ GO Schenck: About autoxidation in the furan series, II. Part: About autoxidation of furan and 2.5-dimethyl-furan . In: Reports of the German Chemical Society 1945 , 77 (9-10), pp. 661-668. doi : 10.1002 / cber.19450770902
- ↑ RM Adams, CA Van der Werf: Condensation of Acetoacetic Ester with Some Unsymmetrical Epoxides . In: J. Am. Chem. Soc. 1950 , 72 (10), pp. 4368-4373. doi : 10.1021 / ja01166a010
- ↑ cobocards.com: Toxicity of hydrocarbons (n-hexane)
- ↑ Deutsche Bundesstiftung Umwelt: Biocatalytic synthesis of chiral synthetic building blocks , accessed on January 28, 2020.
- ↑ Lawrence P. Wackett and C. Douglas Hershberger: Biocatalysis and Biodegradation: Microbial Transformation of Organic Compounds , ASM Press, Wash. DC., 2001 .
- ↑ T. Soriano, M. Menéndez, P. Sanz, M. Repetto: Method for the simultaneous quantification of n-hexane metabolites: application to n-hexane metabolism determination , in: Human & Experimental Toxicology , 1996 , 15 (6), Pp. 497-503; PMID 8793533 .
- ↑ Daunderer-Klinische Toxikologie-50. Erg. Run 1/90.
- ↑ KÜHN, BlRETT: Leaflets Hazardous Working Materials, ecomed, Landsberg, 1986 , Erg. Lfg.
- ↑ Pollutant Lexicon: Aliphatic Hydrocarbons
- ↑ cobocards.com: CBP
- ↑ Philippe Gorlier: SAFETY INSTRUCTIONS 2008 - REPRODUCTION TOXICITY (PDF; 562 kB)