Brodifacoum
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
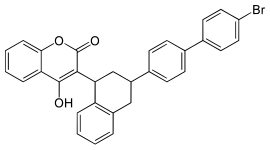
|
|||||||||||||||||||
| Structure without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Brodifacoum | ||||||||||||||||||
| other names |
3- [3- (4'-Bromo-1,1'-biphenyl-4-yl) -1,2,3,4-tetrahydro-1-naphthyl] -4-hydroxycoumarin |
||||||||||||||||||
| Molecular formula | C 31 H 23 BrO 3 | ||||||||||||||||||
| Brief description |
almost white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 523.4 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.42 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
228–230 ° C (mixture of isomers) |
||||||||||||||||||
| solubility |
almost insoluble in water (0.24 mg l −1 ) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Brodifacoum is a synthetically produced, highly toxic chemical compound from the group of 4-hydroxycoumarins . It is a cream- to beige-colored lipophilic , practically insoluble in water, odorless powder. Brodifacoum is used as a rodenticide (rodent poison).
synthesis
Commercial production involves a seven-step synthesis sequence. In the first step, phenylacetic acid chloride is reacted with 4-bromobiphenyl in a Friedel-Crafts acylation . The resulting ketone is first reduced to the alcohol using sodium borohydride , which is then nucleophilically substituted by phosphorus tribromide to form the bromine compound . The fourth step of the synthesis provides a link with the sodium salt of diethyl malonate , the product in the presence of polyphosphoric acid to a 3,4-dihydro-2 H -naphthalene-1-one intermediate is cyclized. A second reduction by means of sodium borohydride gives a 1,2,3,4-tetrahydronaphthalen-1-ol derivative, which is reacted with 4-hydroxychromen-2-one to form the target compound in the last synthesis step. The synthesis sequence gives a mixture of isomers .
A synthesis of pure enantiomers is commercially unattractive because of the small differences in effectiveness of the stereoisomers. Laboratory syntheses of the individual isomers proceed using stereoisomeric starting materials and intermediates.
Chemical properties
Stereochemistry
Brodifacoum is a chiral compound that contains two asymmetrically substituted carbon atoms. This gives four stereoisomers, with the (1 S , 3 S ) and (1 R , 3 R ) isomers and the (1 S , 3 R ) and (1 R , 3 S ) isomers forming corresponding pairs of enantiomers . The melting points for the (1 R , 3 S ), (1 S , 3 R ) isomer pair are 227 ° C. and for the (1 R , 3 R ), (1 S , 3 S ) isomer pair, 224 ° C.
The racemate from (1 S , 3 S ) - and (1 R , 3 R ) form is diastereomeric to the racemate from (1 S , 3 S ) - and (1 R , 3 R ) form. The (racemic) diastereomers can be differentiated on the basis of their 1 H and 13 C NMR spectra . Brodifacoum is commercially available as a mixture of four stereoisomeric molecules that differ in their physiological effects. Certain physical and chemical properties of the four stereoisomers are also different.
Using suitable synthesis strategies (“ asymmetric synthesis ”) or separation processes ( racemate resolution ), the individual stereoisomers can be purposefully produced or isolated.
effect
For birds and mammals as well as humans brodifacoum is very toxic for fish , it is even more toxic. It can be absorbed through the digestive tract (orally) as well as through the skin or the respiratory tract.
Due to the structural similarity of coumarins to vitamin K 1, they cause a competitive inhibition of enzymes that are involved in the formation of the blood clotting factor prothrombin . Thus, they indirectly abolish the blood's natural ability to clot (indirect anticoagulant ) and damage blood vessels (liver). This causes blood fluid to leak through mucous membranes, body cavities and internal organs, causing the victim to bleed to death. Correspondingly, the general symptoms of intoxication typical of anticoagulants are skin and mucous membrane bleeding and, in severe cases, blood in the stool and urine. After the ingestion of a lethal dose, death does not occur immediately, but only after four to five days (in rats) due to exhaustion due to the loss of blood and fluid ( dehydration ).
toxicity
The permitted daily dose ( ADI ) for humans is 0.0000005 mg / kg / day (= 0.5 ng / kg / day) and the NOAEL value is 0.001 mg / kg / day.
The following mean lethal concentrations (LD 50 ) were determined for the mixture of isomers in various mammals :
- Rats (oral): 0.27-0.3 mg / kg body weight
- Mice (oral): 0.4 mg / kg body weight
- Rabbit (oral): 0.3 mg / kg body weight
- Guinea pigs (oral): 0.28 mg / kg body weight
- Cats (oral): 0.25 mg / kg - 25 mg / kg body weight
- Dogs (oral): 0.25 mg / kg body weight
LD 50 values for various birds are between about 1 mg / kg body weight and 20 mg / kg body weight.
LC 50 for fish:
- Trout (exposed to the substance for 96 hours): 0.051 mg / liter.
The following LD 50 values were determined on the mouse for the individual stereoisomers :
- (1 R , 3 R ) -isomer: 0.5-0.8 mg · kg −1
- (1 S , 3 S ) -isomer: 0.4-0.9 mg kg −1
- (1 S , 3 R ) -isomer: 0.4-0.9 mg kg −1
- (1 R , 3 S ) -isomer: 0.5-0.8 mg · kg −1
The effectiveness of the various stereoisomers can thus be regarded as approximately the same.
Countermeasures
Phylloquinone ( vitamin K 1 ) - administered intramuscularly or orally - acts as an antidote to coumarins .
As an immediate measure after ingestion: induce vomiting . Blood transfusions may be needed to counteract blood loss.
use
Use as a rodenticide
Brodifacoum is the most potent rodenticide ("rat poison") available to date and as such is widely used for poisoning rodents , mainly rats and mice , but also rabbits and possums , particularly for predators such as. B. Cats are at risk of secondary poisoning by poisoned animal corpses: Even the consumption of a single poisoned animal can be fatal.
Brodifacoum is used as an active ingredient in bait with a weight percentage of 0.005% and, as a so-called second-generation anticoagulant ("super warfarin "), is lethal even in a single dose, i.e. it also kills animals with a resistance to first-generation anticoagulants (namely warfarin) .
The delayed death of the animals with these means is a desirable property: Since there is no advance warning of conspecifics, they are therefore also in socially intelligent animals such as. For example, rats are effective, as they quickly learn from their conspecifics how to avoid the poison when they use substances that work immediately because of their social intelligence. The slow weakening of the animals also means that they hardly take in any more food and ultimately die with a practically empty gastrointestinal tract, which means that the carcasses develop less odor as they decompose. A legend, however, is that rodents so poisoned would leave human dwellings in search of water and die in the open.
Brodifacoum was used to clear the island of South Georgia from rats introduced by humans.
Brodifacoum found similar application on Lord Howe Island , which belongs to Australia , where rats introduced since a shipwreck in 1918 had wiped out several endemic animal species.
Modification of illegal drugs
In 2018, a scientific article in the New England Journal of Medicine published a case series of drug users who suffered from a tendency to bleed that was difficult to control. All users had ingested synthetic cannabinoids that were contaminated with Brodifacoum and other superwarfarins. Most of the patients survived, but only through massive countermeasures with plasma infusions and vitamin K administration. The cause of the contamination is stated to be that manufacturers of the synthetic cannabinoids try to influence the effectiveness or the action profile by using additional substances. Due to its easy availability and high level of danger, the use of Brodifacoum is also being critically observed by security authorities and the CDC in order to assess its potential as a murderous poison.
Admission
As a biocidal product
According to European legislation (Directive 98/8 / EC on the placing of biocidal products on the market) and with the resolution of February 9, 2010, a decision has been made to add the active ingredient Brodifacoum to the corresponding list (Appendix I / IA of Directive 98/8 / EC ) for product type 14 (rodenticides). The sale of biocidal products that contain the active ingredient Brodifacoum is therefore still permitted in the EU (Switzerland has adopted this provision). However, this permit was subject to certain conditions:
- The nominal concentration of the active ingredient in the products must not exceed 50 mg / kg and only ready-to-use products may be approved. The products must not be used as an adhesive poison.
- Appropriate measures must be implemented to reduce the risk to humans, non-target animals and the environment. For example, the restriction to use by specialist staff, the definition of a maximum package size and the obligation to use secure, stable bait boxes.
- The approval is initially limited to January 31, 2017, an extension of this period is linked to a new risk assessment.
As a pesticide
In accordance with European legislation (Directive 91/414 / EEC of July 15, 1991 on the placing of plant protection products on the market) and with a resolution of June 21, 2007 of the EU Commission, it was decided not to include the active ingredient Brodifacoum in Annex I of Directive 91/414 / EEC (positive list of the active substances permitted in plant protection products). The sale of plant protection products with the active ingredient Brodifacoum has not been allowed in the EU since 2011.
In Switzerland, the active ingredient Brodifacoum is no longer approved as of May 15, 2012 according to the amendment to the Plant Protection Ordinance.
See also
The substance is closely related to the somewhat less toxic bromadiolone used for the same purposes .
Individual evidence
- ↑ a b c WHO / FAO Data Sheet on Pesticides (PDS) for Brodifacoum ( Memento from April 11, 2015 in the Internet Archive )
- ↑ a b c d Entry on Brodifacoum in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on 4-hydroxy-3- (3- (4′-bromo-4-biphenylyl) -1,2,3,4-tetrahydro-1-naphthyl) coumarin in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Brodifacoum data sheet at Sigma-Aldrich , accessed on May 13, 2017 ( PDF ).
- ↑ RS Shadbolt, DR Woodward, PJ Birchwood: Synthesis of Some Tetrahydronaphthyl- and Flavanyl-coumarins. In: J. Chem. Soc. Perkin Trans. I, 1976, pp. 1190-1195, doi: 10.1039 / A607269K .
- ^ RT Hadler, RS Shadbolt: Ger. Patent 2424806, 1975; U.S. Patent 3957824, 1976; US Patent 4035505, 1977.
- ↑ a b c d P. S. van Heerden, BCB Bezuidenhoudt, D. Ferreira: Efficient Asymmetric Synthesis of the Four Diastereomers of Diphenacoum and Brodifacoum. In: Tetrahedron . 53, 1997, pp. 6045-6056, doi: 10.1016 / S0040-4020 (97) 00254-8 .
- ↑ J.-C. Jung, O.-S. Park: Synthesic Approaches and Biological Activities of 4-Hydroxycoumarin Derivatives. In: Molecules. 14, 2009, pp. 4790-4803, doi: 10.3390 / molecules14114790 .
- ↑ PS van Heerden, BCB Bezuidenhoudt, D. Ferreira: Improved synthesis for the rodenticides, diphenacoum and brodifacoum. In: J. Chem. Soc. Perkin Trans. 1, 1997, pp. 1141-1146, doi: 10.1039 / A607269K .
- ↑ JR Cort, H. Cho: 1 H and 13 C NMR chemical shift assignments and conformational analysis for the two diastereomers of the vitamin K epoxide reductase inhibitor brodifacoum. In: Magn. Reson. Chem. 47, 2009, pp. 897-901, doi: 10.1002 / mrc.2475 .
- ↑ VV Shkarenda, PV Kuznetsov: Current state of the liquid column chromatography of coumarins. In: Chemistry of Natural Compounds. 29, 1993, pp. 137-150, doi: 10.1007 / BF00630102 .
- ↑ WHO Specifications and Evaluations for Public Health Pesticides: Brodifacoum. (PDF; 151 kB) WHO , p. 19 , accessed on June 10, 2017 (English).
- ↑ Poisons Information Monograph (PIM) for Brodifacoum , accessed December 9, 2014.
- ↑ D. Kaukeinen: Experimental rodenticide (Talon) passes lab tests; moving to field trials in pest control industry. In: Pest Control. v. 47 (1), Jan 1979, pp. 19-21, 46.
- ↑ Brodifacoum (Talon, Havoc) - Chemical Profile 1/85 on cornell.edu
- ↑ Selleys: MSDS Data Sheet for Talon Rat and Mouse Killer
- ↑ Elaine C. Murphy, B. Kay Clapperton, Philip MF Bradfield, Hazel J. Speed: Brodifacoum residues in target and non-target animals following large-scale poison operations in New Zealand podocarp-hardwood forests. In: New Zealand Journal of Zoology. 25 (4), 01/1998, pp. 307-314. doi: 10.1080 / 03014223.1998.9518160
- ↑ Health and Safety Guide (HSG) for Brodifacoum , accessed on December 1, 2014.
- ↑ Safety data sheet AMB-Vertriebs GmbH. (PDF file; 150 kB).
- ↑ Entry on Brodifacoum. In: Römpp Online . Georg Thieme Verlag, accessed on June 16, 2014.
- ^ Matt Ridley: The success of a bold bid to rid a subantarctic island of rats and deer
- ↑ Amar H. Kelkar, Nichole A. Smith, Annia Martial, Harsha Moole, Michael D. Tarantino: An Outbreak of Synthetic Cannabinoid – Associated Coagulopathy in Illinois . In: New England Journal of Medicine . tape 379 , no. 13 , September 27, 2018, ISSN 0028-4793 , p. 1216-1223 , doi : 10.1056 / NEJMoa1807652 , PMID 30280655 .
- ↑ Directive 98/8 / EC of February 16, 1998 on the placing of biocidal products on the market . In: Official Journal of the European Communities . L, No. 123, April 24, 1998, pp. 1-63.
- ↑ Directive 2010/10 / EU of 9 February 2010 - Decision amending Directive 98/8 / EC to include brodifacoum in Annex I of . In: Official Journal of the European Communities . L, No. 37, February 10, 2010, pp. 44-46.
- ↑ Directive 91/414 / EEC of July 15, 1991 on the placing of plant protection products on the market . OJ L 230, August 19, 1991, pp. 1-32.
- ^ Decision of June 21, 2007 (2007/442 / EG) on the non-inclusion of certain active substances in Appendix I and revocation of the approvals for plant protection products . In: Official Journal of the European Communities . L, No. 166, June 28, 2007, pp. 16-23.
- ↑ CH: Amendment to the Ordinance on Plant Protection Products (PDF file; 484 kB). SR 916.161 - Amendment of April 21, 2011.





