Diethyltoluamide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Diethyltoluamide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 12 H 17 NO | ||||||||||||||||||
| Brief description |
colorless liquid with a faint odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 191.27 g · mol -1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.996 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−45 ° C |
||||||||||||||||||
| boiling point |
240 ° C |
||||||||||||||||||
| Vapor pressure |
0.01 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.5212 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Diethyltoluamide ( DEET ) is a chemical insect repellent . It has a wide spectrum of action on various insects , but it can cause allergies and should not be used by pregnant women, breastfeeding women and children under two years of age.
DEET was developed by the US Army in 1946 as an insect repellent for military use. It was used in the military in regions with a high incidence of mosquitoes , including Southeast Asia , e. B. in the Vietnam War . In 1957 the substance was approved for civil use and marketed commercially from 1965.
Occurrence and representation
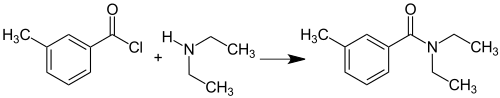
DEET was found in nature in female palm moths of the species Pectinophora gossypiella . Industrially, N , N diethyl m -toluamid from the acid chloride of 3-methylbenzoic acid and diethylamine prepared:
Mechanism of action
The question of the mechanism of action has not yet been finally clarified. An older study suggested that DEET achieves its effect by blocking the general odor receptor (Orco), which means that the insects are less able to perceive substances such as lactic acid or carbon dioxide in order to detect a victim . However, recent work has shown that insects can smell DEET and thus stay away. The animals perceive DEET both through olfactory receptors and through their taste receptors.
Toxic effect
DEET irritates the eyes and mucous membranes, but usually not the skin. In rare cases, the substance can also cause skin irritation and epileptic seizures (1 case per 100 million DEET users, according to the Environmental Protection Agency ). According to some studies, DEET can increase the incidence of insomnia, impaired cognition, and mood swings. In high-risk areas, protection against malaria and other mosquito-borne diseases is a priority, including for young children.
A French research group reported in 2009 that DEET inhibits the enzyme acetylcholinesterase . This enzyme breaks down the neurotransmitter acetylcholine . In combination with insecticides , the effect of the DEET increases.
use
DEET is marketed in Europe under the trade names Care Plus Anti-Insect DEET (up to 50%), OFF , Parazeet and Nobite (50% share) as well as the product Anti Brumm Forte (30% share). In the Autan products, it has now been replaced by the better-tolerated and also effective against ticks, Icaridin , for which a patent was applied for in 1988. The active ingredient DEET has only a very low odor and is mixed with various fragrances in commercially available sprays.
When using it, it should be noted that DEET - because it is a solvent - can attack some plastics , synthetic fibers and leather . When using DEET-containing repellants on young children and pregnant women, caution is advised - due to the insufficient and sometimes contradicting data - and the instructions for use must be observed.
Under the EU-test program of biocide - existing substances DEET is for use in the product type 19 ( repellents and attractants ) and 22 ( liquids for embalming and Taxidermie evaluated).
Web links
- Entry on N, N-diethyl-meta-toluamide in the Consumer Product Information Database
Individual evidence
- ↑ Entry on DIETHYL TOLUAMIDE in the CosIng database of the EU Commission, accessed on December 28, 2019.
- ↑ a b c d e f g h i j Entry on N, N-diethyl-m-toluamide. In: Römpp Online . Georg Thieme Verlag, accessed on March 16, 2011.
- ↑ a b c d e Entry on N, N-diethyl-m-toluamide in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on N, N-diethyl-m-toluamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on diethyltoluamide in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Jörn Köhler: Galenical development of an insect repellant with improved adhesive strength , dissertation 2001, Rheinische Friedrich-Wilhelms-Universität Bonn; urn : nbn: de: hbz: 5n-00336 .
- ↑ California Department of Pesticide Regulation: DEET Risk Characterization Document (PDF file; 2.9 MB).
- ↑ a b Technical information on DEET from the US National Pesticide Information Center (NPIC) (PDF file; 314 kB).
- ^ A Unified Nomenclature System for the Insect Olfactory Coreceptor . Chem. Senses (2011) 36 (6): 497-498; doi: 10.1093 / chemse / bjr022 .
- ↑ Science 319 (5871): 1838-1842: Insect Odorant Receptors Are Molecular Targets of the Insect Repellent DEET .
- ↑ www.physorg.com: Groundbreaking research shows DEET's not sweet to mosquitoes .
- ^ Mosquitoes smell and avoid the insect repellent DEET . PNAS 2008 vol. 105 no. 36; doi: 10.1073 / pnas.0805312105 .
- ↑ A natural polymorphism alters odor and DEET sensitivity in an insect odorant receptor . Nature 478: 511-514 (Oct. 27, 2011); doi: 10.1016 / j.neuron.2010.07.006 .
- ^ Avoiding DEET through Insect Gustatory Receptors . Neuron Volume 67, Issue 4, 2010, 555-561; doi: 10.1038 / nature10438 .
- ↑ Vincent Corbel et al .: Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent deet . BMC Biology 2009, 7:47 am (free full text access).
- ^ Insect Repellents: Principles, Methods, and Use. Eds. Debboun, M., Frances, SP, Strickman, D. CRC Press, Taylor & Francis Group Boca Raton Florida.
- ↑ European patent EP 281908 from March 2, 1988.
- ↑ Autan website: start page. Retrieved April 3, 2018.
- ↑ Mirko Altenkämper, Martin Schlitzer: Repellants and insecticides: Successful against the insect attack , Pharmazeutische Zeitung , edition 11/2008.
- ↑ Official Journal of the European Union: COMMISSION REGULATION (EC) No. 1451/2007 of December 4, 2007 on the second phase of the ten-year work program according to Article 16 (2) of Directive 98/8 / EC of the European Parliament and of the Council on placing biocidal products on the market (PDF) .