Iron smelting among the Teutons
Little research has been done on the beginnings of iron ore smelting among the Teutons . The oldest evidence, however, dates back to the "Younger Pre-Roman Iron Age". The Iron Age , named after the use of metal, begins in Central Europe in the 8th century BC. And is divided into:
- the Early or Older Iron Age (800–450 BC) or the Hallstatt Period
- the Late or Younger Pre-Roman Iron Age (450 BC - end of the 1st century BC) or the Latène Age
- In Northern Europe, the Roman Empire (0–375 AD) is not followed by the Migration Period, as in Central Europe, but the so-called “Germanic Iron Age” (375–650 AD), which in Sweden began with the Vendel Period (650–800 AD). Chr.) And in Denmark even ends with the Viking Age (800–1050 AD).
First iron
As a metal, iron has been around since the 6th millennium BC. Known as individual jewelry items from the Middle East ( Samarra , in Iraq ). Reliable evidence of the use of iron comes from the 4th millennium BC. Chr., E.g. B. from Ur in Mesopotamia or from El Gerzeh and Armant in Egypt . However, the finds cannot yet be linked to smelting . Their sometimes high nickel content indicates the use of meteorite irons . The first written mention of iron comes from 2300 BC. In the high culture of the Sumerians . The beginnings of iron production using the racing fire process lie in the last quarter of the 2nd millennium BC. In Anatolia and neighboring countries. The process produces malleable iron, which is clearly superior to the copper alloys previously used in terms of hardness and toughness. The oldest iron find in Central Europe dates from 1700 BC. And took place in Slovakia . 1200 BC First hardened iron, i.e. steel , appears. During the 9th century BC Knowledge of the racing fire process came to Central Europe, where it remained the only process for iron extraction until the 16th century AD.
Iron production
First evidence
As part of a project funded by the German Research Foundation (DFG), research is being carried out into iron ore smelting in the pre-Roman Iron Age in northern Central Europe. In 2009/10 the oldest racing fire stoves in northern Central Europe were found in Glienick ( Zossen ). Radiocarbon dates refer to the 4th / 3rd Century BC In Brunn, district of Mecklenburg Lake District, Groß Siemz, district of Northwest Mecklenburg and Vietow, district of Rostock, well-preserved remains of Rennofen from the centuries before the turn of the century were uncovered for the first time in Mecklenburg-Western Pomerania .
In Dalheim , on the outskirts of Wetzlar , at least five multi-period settlement sites with iron ore smelting and forging activities were located in a very small space. The chronological framework ranges from the younger Iron Age (4th – 1st century BC) through the Roman Empire (1st – 4th century AD) to the early and high Middle Ages (5th – 12th centuries). Century). In 2006, on the occasion of the extensive excavations and the spectacular finds near Wetzlar-Dalheim, an "Open Excavation Day" took place. In various experiments bronze was cast and iron was forged. A highlight was the replica and driving of a racing furnace with tapping of the still iron-containing slag.
The extraction of iron was of great importance to the Germanic peoples of the Roman Empire (1st – 4th century AD). The most important devices, weapons and tools were made of iron. The common and abundant lawn iron stone , which, according to K. Fiege, formed from the Atlantic Ocean served as raw material . Lawn iron stone is an easily degradable iron compound that forms naturally within a short time in the upper soil layers under changing groundwater levels or under waterlogging. The traces of iron ore smelting are marked on agricultural land by a concentration of lead to black-gray pieces of iron slag. In wastelands or forest areas they are preserved as iron cinder hills with mostly sparse vegetation. They can be found in large numbers on the wide sand areas of the Mittelrücke in Schleswig-Holstein . Areas of great density of finds coincide with the distribution areas of the lawn iron stone deposits. How good the raw material sources must have been can be estimated from the deposits that were mined up to modern times. Today amelioration and modern agriculture prevent a considerable new formation of this ore. Of the more than 200 known lawn iron stone deposits in Lusatia , only a tiny part could be smelted, as the iron content or thickness are too low.
smelting
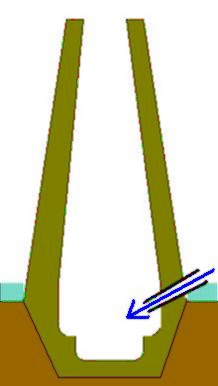
The ore was smelted in simple cylindrical to conical chimney-like shaft furnaces made of clay, which, depending on the type of construction, were also built over a deep hearth or slag drainage pit . The process was comparatively complex. Wood and charcoal were used as heating material . Openings in the lower furnace area provided the required oxygen due to the chimney effect (so-called 'self-drawing' furnaces). Alternatively, bellows were connected for a regulated air supply. The smallest ovens were around 1.2 m high and 0.6 m in diameter. The aim of smelting was the extraction of iron by direct reduction from the iron oxide of the ore. This included separating the slag from the low-carbon billet at around 1,150 ° C to make malleable steel. The prerequisites for successful smelting include a process temperature of at least 150 to 1200 ° C. The iron molecules contained in the glow of welding and reduced from the ores sintered in a viscous slag resulting from the admixtures of the ores (gangue) into smaller or larger iron lumps without melting. In the smelting process, carbon monoxide (CO) was created in the racing furnace through incomplete combustion. This withdrew the oxygen from the iron-containing part of the ore (direct reduction), whereby metallic iron was formed and non-metallic components of the ore (the so-called gangue ) were melted and drained as slag through tapping openings. The glowing slag ran out of the furnace, which gave the 'racing furnace' its name. The oxygen is broken down from trivalent iron (Fe 2 O 3 ) via magnetite (Fe 3 O 4 ) and wustite (FeO) to metallic iron. The reducing agent is the carbon monoxide (CO) of the CO / CO 2 gas mixture that is produced when the charcoal is burned or, in the case of direct contact, the solid carbon of the charcoal. Lawn iron stone is a subsequently solidified sediment fraction of the soil and not ore in the geological sense. Besides iron (III) oxide (Fe 2 O 3 ), it consists mainly of iron hydroxide (2Fe 2 O 3 • 3H 2 O or mineralogically limonite ), silica (SiO 2 ) and contains admixtures of MnO, CaO, Al 2 O 3 , SO 2 and P 2 O 5 , whereby only MnO and P 2 O 5 have a stronger influence on the reduction process. Racing fire stoves were one-way stoves. After the smelting, the furnace usually had to be smashed to get to the rag. In the best case scenario, archaeologists will therefore find the cinder blocks in the hearth pits. Fragments of the furnace shaft and ceramic nozzle fragments made from baked clay are rarer. Mostly, however, only remnants of the racing fire slag are found. The slag was removed from the slag by repeated heating in ovens and hammers on stone anvils and welded together to form larger pieces of iron from which iron devices could be forged.
Smelting sites
In the Oberlausitz about 50 slag sites is known. 26 of them as smelting sites. Several sites have been found in and around Wetzlar and in the entire Lahn-Dill area . However, the conditions only partially allow an exact dating. Smelting sites can contain racing furnaces, bake-out stoves, shallow forge pits, kilns and roasting areas. In Schleswig-Holstein, 20 to 25% of the datable slag finds can be classified in the pre-Roman Iron Age. 7 to 8% of this relates to the older phase of the period. 55 to 58% of the finds belong to the Roman Empire and the Migration Period. The remaining sites (around 14.5%) are from the Middle Ages. There is no evidence of smelting in Lusatia during the early Iron Age (8th – 5th centuries BC). Apparently iron was only imported at that time. The majority of the sites (e.g. Altliebel , Burgneudorf , Merzdorf , Milkel , Weißkollm , Jahmen , Jänkendorf , Lomske) are Germanic (2nd - 4th century AD) with a focus on the late Roman Empire (3rd century AD) . – 4th century AD). Some sites belong to the Slavic early Middle Ages. To date, over 300 smelting sites have been discovered in the Saar coal forest between Neunkirchen and Saarbrücken , but these could not be precisely dated.
The smelting sites are often on the edge of terraces over river valleys and on slight elevations. Because of the emissions , they were mostly built away from settlements. The ore and the wood for the coal usually came from the immediate vicinity. Smelting sites were only used seasonally, but sometimes for several years. The size of the workplaces was based on the number of ovens and generally fluctuates between 10 and over 60 oven systems and between 250 m² and two hectares. The largest Germanic smelting site in Germany was at Wolkenberg in Niederlausitz, where over 1200 racing furnaces were found on an area of around 220 hectares. The iron ore deposits were so heavily mined that K. Fiege looked in vain for good iron ores in the area in Schleswig-Holstein, which is particularly suitable for the formation of ore deposits. Judging by the findings, the imperial smelting area on the Neumünster Sander can be ascribed the character of an early commercial center, in which iron ore smelting must have been the livelihood for the residents. In Schleswig-Holstein the following explanation of the findings can be offered: Since the beginning of the pre-Roman Iron Age, the ore deposits in Mittelholstein, which are located near the repeatedly visited smelting areas, have apparently been mined so heavily that the medieval ironworks were forced to move the more difficult to access ore deposits in the area to dismantle the heavily waterlogged low-lying areas remote from traffic.
Germanic Rennofen method
The thermal process can be divided into three phases.
- In the preheating phase, the furnace is brought to process temperature.
- In the charging and smelting phase, either a mixture of charcoal and ore (ratio approx. 1: 2 to 1: 3) or the two components are placed in alternating layers in the furnace. This process is repeated several times.
- The final post-heating phase covers the period from the last filling to the end of the oven cycle. From the cinder blocks in the hearth pits, which weigh up to 100 kg, it becomes clear that, depending on the size, a kiln could extend over several days. The consumption of ore and charcoal naturally exceeded the weight of the slag and pellet produced many times over. The iron yield depended on the quality of the ore (at least 65% FeO) and the duration of the furnace cycle - in principle, one can assume an efficiency of 25 to over 30% if current experimental archaeological experiments are used.
Roast
The lawn iron stone was enriched with iron oxide (Fe 2 O 3 ) by roasting before it was smelted . By heating the ore chunks in an open fire to a few 100 ° C, iron hydroxide is dehydrated to iron oxide and disruptive organic components and some of the sulfur in the ore (particularly high content in lawn iron ore) is removed. Roasting reduces the strength of the ore to such an extent that it can be more easily crushed into the required grain sizes (2–6 mm). Inferior parts / parts with a high proportion of sand can be separated more easily.
Steel quality
The quality and workability of the iron produced in the racing furnace is determined not only by the very low proportions of manganese (Mn), sulfur (S), silicon (Si) and phosphorus (P), but above all by the carbon content (C). The warmer the pellet gets in the furnace (e.g. near the air nozzles), the higher the carbon content of the iron. This can go so far that cast iron is produced in small quantities, which cannot be forged and has therefore been discarded. The parameters have an influence on the achievable temperature:
- Calorific value and mass of charcoal,
- (supplied) air volume
- the cross-sectional area of the furnace at the level of the air inlet
- the thermal insulation of the furnace wall.
With optimal interaction of the components, temperatures of over 1300 ° C can be achieved. However, these peak values are only present in the immediate area of the air inlet openings. In principle, pig iron could be produced in the racing furnace with a sufficient supply of air and correspondingly high temperatures. However, pig iron has a carbon content of more than 2.06%. It is neither hot nor cold deformable and would have to be laboriously decarburized in order to produce forgeable iron / steel. Steel is an iron that can be forged without reservation, with a carbon content of up to approx. 1.7%. Low-melting slag has been found throughout the smelting sites, which mineralogically mainly consists of fayalite (Fe 2 SiO 4 ) and wüstite ("FeO"). Fayalite has a melting point of 1200 ° C, which was further reduced by the proportions of CaO, Al 2 O 3 and P 2 O 5 .
The aim of the Germanic metallurgists was to produce a low-carbon, malleable iron shell. Only temperatures of up to 1200 ° C were necessary for this. The high iron content of the slag of 55 to 70% makes it clear that only a small part of the iron oxide is reduced to iron during smelting. The major part forms a silicate slag with other parts. Therefore, only high quality turf iron stones and ores could be smelted for iron production (> 65% FeO). Despite the air flow and fan, there are very large temperature differences in the racing furnace and relatively small areas in which the temperatures of over 1150 ° C required for slag formation are reached. In the upper furnace shaft, the iron oxides are reduced to FeO and only in small quantities to metallic iron.
See also
- Racing furnaces from Brunn, Groß Siemz and Vietow
- History of production technology
- Metallurgy "History" section
- Technology in antiquity
literature
- K. Fiege: The lawn iron ores of Schleswig-Holstein . New Yearbook for Mineralogy 1950, 219 ff.
- H. Hingst: The pre-Roman iron age. In: History of Schleswig-Holstein , Vol. 2 (1964) 233 ff.
- H. Hingst: Prehistoric iron smelting sites on the Neumünsteraner Sander. In: Rust-Festschrift 1968
- Hauke Jöns : At the beginning of iron smelting in the north - the racing fire furnaces from Groß-Siemz, Vietow and Brunn . In: Uta Maria Meier (Red.): The A20 motorway - Northern Germany's longest excavation. Archaeological State Museum and State Office for Land Monument Preservation Mecklenburg-Western Pomerania, Lübstorf 2006, ISBN 3-935770-11-1 , pp. 97ff.
- Markolf Brumlich, Michael Meyer : Furnace systems from the pre-Roman Iron Age near Waltersdorf, Dahme-Spreewald district. A contribution to early iron smelting . In: Insights. Archaeological contributions for the south of the state of Brandenburg 13, 2004 / work reports on the preservation of soil monuments in Brandenburg 13, 2004, pp. 167–196.
Individual evidence
- ↑ As Germanic archeology mostly groups living in north-central Europe from the Jastorf culture (600 BC) onwards. W. Künnemann: Jastorf - history and content of an archaeological concept of culture. In: The customer. NF 46, 1995, 61-122
- ↑ "Early iron in the low mountain range": Iron production on the central Lahn from the Latène period to the Middle Ages. In: uni-bamberg.de , October 27, 2016, accessed on May 30, 2017
- ↑ http://www.denkmalpflege-hessen.de/Download/TagderoffenenGrabung_B49-1.pdf
Web links
- Iron among the Teutons. Experiment of the Museum of West Lusatia
- Iron smelting in the pre-Roman Iron Age of northern Central Europe. The case study of the Teltow. Institute for Prehistoric Archeology at the Free University of Berlin