Evolutionary developmental biology
The evolutionary developmental biology or short evo-devo (derived from the term evolutionary developmental biology ) is a discipline of biology , which, as is the control of the individual development of living things (studied ontogeny ) in the evolutionary history has developed.
A group of philosophers and theoretical biologists (with the program of an extended synthesis of the theory of evolution ) also uses the term Evo-Devo as a term for research on the question of whether and how the processes of embryonic development may influence the evolution of organisms ( phylogenesis ).
Although evolutionary developmental biology already played an important role in the formation of theories in the 19th century, a significant experimental basis for a well-founded further development of the theory did not emerge until the 1980s with the increasing clarification of embryonic development through the discovery of control genes and their mechanisms of action Products.
Since then, developmental and molecular biological laboratory methods have been used to determine which factors and control mechanisms are responsible for the formation of tissues and organs. The question of how this control can be reconstructed as a result of the course of the phylogenetic history of the organisms is factually linked to this. On the theoretical as well as the experimental level, there is inevitably an integration of developmental biology and evolutionary biology.
An essential source of knowledge is the decoding of the genetic basis for numerous development processes that were completely puzzling until the 1980s, which began with the discovery of the so-called Hox genes .
As an overall statement and extension of the Darwinian and synthetic theory of evolution, EvoDevo can introduce that in the mechanism of the interplay of random variation and natural selection there is much more system (development) and less randomness in the emergence of phenotypic variation.
History of the study of evolutionary changes in ontogeny
Darwin and the 19th Century
Charles Darwin was already aware that embryonic development is relevant to evolution . In his time, however, it was neither possible to examine the developmental processes in more detail, nor were the exact mechanisms of inheritance or genes and DNA known. Darwin therefore focused on natural selection as the primary factor in evolution .
In addition to Darwin, there are a few embryologists from the 19th century who also dealt with evolutionary aspects:
- Karl Ernst von Baer found in vertebrates that embryos of different species are more difficult to distinguish the earlier in their development they are found ( Baer's rule )
- Fritz Müller combined natural selection and embryology in his book For Darwin (1864) and demonstrated in the development phases of crustaceans that their tribal history could not be explained without Darwin's theory. His studies were as inspiring as those of Baers
- Ernst Haeckel . The biogenetic basic rule , which is no longer in use today, goes back to him , which, in sharpening a recapitulation thought that was widespread at the time, indicates that the parallels observed between ontogenesis and phylogenesis of organisms are based on the embryonic repetition of characteristics that in the phylogenetic history of species were Stage were trained.
- Wilhelm Roux was a student of Haeckel and the founder of development mechanics . He was already of the opinion that no finished blueprint is inherited ( preformation theory ), but that "the individual cells have a certain leeway within which the events mutually regulate themselves" (1881). From this thought epigenetic ideas were strengthened, as they were previously accepted by Caspar Friedrich Wolff (1734–1794).
Synthetic theory of evolution since 1930
In the last decades of the 19th and the first of the 20th century, Darwin's findings were incorporated into the new field of comparative embryology. Discoveries such as that of the germ layers (endo-, meso-, ectoderm) were essential for deciphering the homology of the body plans. After the great discoveries of the early days, however, the vigor slackened when differences of opinion broke out between renowned researchers about the importance of numerous details that could not be determined with the methods of the time. The main interest of research then turned to new disciplines such as developmental mechanics and genetics.
Even during the emergence of the synthetic theory of evolution in the 1930s and 1940s, there were a few scientists who tried to focus more on development (e.g. Richard Goldschmidt , Conrad Hal Waddington , Iwan Iwanowitsch Schmalhausen ). The synthetic theory of evolution, however, with the dominant foundation of population genetics ( Ronald Aylmer Fisher , Sewall Wright , JBS Haldane ) with the help of other disciplines (including zoology , systematics : Ernst Mayr ), was strongly oriented towards statistical-descriptive thinking, so that principles of individual development do not exist Found inclusion in the canon. Thomas Hunt Morgan , one of the early proponents of the synthetic theory of evolution, himself also an embryologist, made the claim in 1932 that genetics was the only scientifically valid approach to the study of evolution. Ideas that dealt with the direct effect of environmental influences on the developing organism (epigenetics) were not pursued further because they contradicted the neo-Darwinian dogma that no information flow is possible that has an external effect on the DNA and changes it in a hereditary manner ( Weismann -Barrier ). Against this background, it can be understood that a researcher such as Conrad Hal Waddington, who theoretically described an environmental induction of developmental changes and the channeling of developmental processes in 1942 and is therefore considered an important precursor of evolutionary developmental biology, was not taken into account by the synthetic theory of evolution. Waddington's theses only experienced a renaissance in the past two decades.
The synthetic theory of evolution , the standard model of today, goes back to Darwin 's theory of evolution , sees the sequence of random and systematic (in sexual reproduction: Recombination) variations , natural selection and the resulting adaptation of populations as sufficient to explain the emergence of organismic diversity. Its representatives, primarily from the 1930s to 1950s, made some restrictive restrictions compared to Darwin, which resulted from the newly discovered genetics. The following basic assumptions of the synthetic theory are important here:
- Gradualism is the assumption that evolutionary developments always take place in continuous, small changes that add up to larger ones. (The concept of gradualism originally goes back to Charles Darwin himself).
- The Weismann barrier . Accordingly, there is no possibility of an inheritable influence on the genome or the germ cells through individual experience of the organisms.
Researchers such as Mary Jane West-Eberhard , Marc Kirschner , Gerd B. Müller and others are currently trying to modify and develop these assumptions.
Constraints and heterochrony
Since the 1980s, there has been an increasing number of voices calling for greater attention to development for evolution ( Stephen Jay Gould et al.): The developmental restrictions that limit the range of evolutionary variation ( constraints ) were increasingly examined . Other researchers dealt with the time shifts of the modular components in the development process ( heterochrony ). B. Shifts in the proportions between different organs could be explained. As early as 1954, Gavin de Beer had proposed that changes in the timing of developmental events can cause the variation of characteristics, such as longer or shorter legs or the formation or non-formation of a tail.
Master control genes and gene regulation
According to Scott F. Gilbert, 1977 can be seen as the year of the conception of the new Evo-Devo research direction, justified by the appearance of three important publications that year: Stephen J. Goulds Ontogeny and Phylogeny , Francois Jacobs Evolution by Thinkering and a technical work by A. Maxam and Walter Gilbert on DNA sequencing . In 1982/83, important master control genes were discovered that are involved in the regulation of basic body building plans, including the Hox genes , which are mainly responsible for the specification of the longitudinal axis of the body (in developmental terminology: the anterior-posterior body axis), and later the Pax- Genes fundamental to eye development, as well as the Mkx genes involved in heart formation. It turned out that the group of Hox genes “occurs in a modified form in all the multicellular animals examined so far”, they are homologous and must therefore be conserved in evolution over a very long period of time; at least since the " Cambrian Explosion " 530 million years ago, Paul Layer even speaks of around a billion years. The discovery of the Hox genes and their homology for the animal phyla is one of the most outstanding discoveries in modern biology in recent decades. (see also homeobox ).
As a result, the ever simpler, faster and cheaper sequencing of genomes and comparative genetics enabled improved insight into the gene regulation processes during development. As a result, this topic developed into one of Evo-Devo's strongest research fields.
Significance for the theory of "extended synthesis"
General
At the time of fertilization, the phenotypic form of an individual is not yet given; the genome (DNA) is "translated" into the form (the phenotype) by processes during development. The embryo must first generate its shape from a single, undifferentiated cell in individual development (ontogeny).
Because every hereditary change in the phenotype can only take place via the change in the embryonic development and this has its own laws, development is a central key for the causal understanding of organismic evolution for the representatives of Evo-Devo. From the point of view of numerous representatives of this research direction, embryonic development can produce spontaneous variation and innovation. The selection only then acts on these forms of variation and selects the most suitable individuals. According to this point of view, morphological form and complex structures (body plans) arise primarily from system-immanent, self-regulating modifications of the organism during embryonic development.
Epigenetic Mechanisms
According to the synthetic theory, evolution is defined as the change in allele frequency ( gene frequency ) in populations . However, this does not explain how variation and innovation arise. While embryonic development was not considered relevant for evolution by the main proponents of synthetic theory, it is now the focus of causal evolution research. While the current theory focuses on the survival of the fittest , evolutionary developmental biology tries to explain the arrival of the fittest, i.e. the question: how do the most suitable ones come about? Evo-Devo expands the research horizon of the theory of evolution to include new questions and new explanations. The epigenetic mechanisms of the reaction abilities of development newly described by Evo-Devo are u. a .:
- Ducting (see Chapter 3 , Chapter 6 and 9.1)
- Heterochrony (see chapter 3 )
- Conserved cellular core processes that can be combined adaptively (see Chapter 5 ):
- weak regulatory linkages in cell-cell communication
- exploratory processes / behavior
- Compartmentalization
- Responsiveness of development to initiating environmental stressors (see Chapter 3 to Chapter 6 and Chapter 8 )
- non-linear effects of development with discontinuous, phenotypic output (see Chapter 4 , Chapter 6 and Chapter 8 ).
For Evo-Devo research, such mechanisms represent, in addition to selection and the genetic factors of mutation , recombination and genetic drift, independent evolutionary factors that allow the development of variation and innovation to be explained mechanistically.
Evo-Devo research topics
Evolutionary developmental biology regards the entire system of embryonic development itself as an evolved system and sees it in a complex systemic connection with the environment. Gerd B. Müller divides the research area into the following three thematic blocks, some of whose individual questions are only at the beginning of scientific work (see Fig. 1):
First of all, as a first block, the evo-devo questions, which are directed from evolution to development:
- The first topic deals with how development in recent species could arise in evolution. What we see and analyze today in more highly developed species in the embryonic phase, this process that has not yet been fully understood in its interaction with the outside world, was not always present in this (as Müller calls it) routine , finely adjusted interplay. There must have been a long evolutionary process until today. Starting with the first metazoa , the selective fixation and genetic routine in the robust forms of development and in the reliable Mendelian forms of inheritance , as we observe them in organisms that exist today, only emerged much later (Gerd B. Müller).
- On the one hand, the evolution of the developmental repertoire means the genetic tools. Sean B. Carroll speaks of the genetic toolkit . One asks and researches how this could arise and evolve or how, for example, genetic redundancy, new gene functions, modularity at the genome level could arise. On the other hand, the development repertoire also includes a complex variety of epigenetic processes (see Fig. 3: The integrated evolutionary development system ). These processes were simpler hundreds of millions of years ago. Today they contain sophisticated, well-coordinated mechanisms that regulate cell interactions, for example. The development repertoire itself was created over millions of years of evolution. According to Müller, it itself multiplies in evolution.
- The question: How does evolution affect special development processes? There are z. B. the heterochrony , the temporal shift of development processes. "Evolutionary modifications in the segmentation and regional differentiation of larger body sections is accompanied by shifts in domains of the Hox gene expressions".
The second block concerns DevoEvo questions that are directed from development to evolution, so to speak, the counter-questions to the first block. These questions are specifically new in Evo-Devo, which make the causal interactions between development and evolution visible and which change the prevailing theory of evolution.
- How does development affect phenotypic variation? In order to prevent undesirable evolutionary variations that are too large from occurring in development, development constraints have formed. Such constraints are physical, morphological and phylogenetic in nature. They lead to a channeling of development ( Conrad Hal Waddington ), to robustness. One also speaks of the development reaction norm , a range within which phenotypic plasticity can take place.
- What does development contribute to phenotypic innovation? If selection alone cannot create a form, there must be another way in which organismic innovation arises. For Evo-Devo, the answer can only lie in development.
- How does development affect the organization of the phenotype? The question of the organization of the body's blueprints in development is not directed towards the emergence or variation of certain body characteristics, but rather how the organism can be produced as an integrated system .
Finally, the third block, that is the Eco-Evo-Devo questionnaire, which concerns the causal relationship between development and evolution with the environment, also newly introduced by Evo-Devo, since the synthetic theory of evolution can not explain such mechanisms of action.
- How does the environment interact with development processes?
- How do environmental changes affect phenotypic evolution?
- How does evolutionary development affect the environment?
A central concept of this set of questions is phenotypic plasticity . Plasticity means that a genotype can produce different phenotypes, which may differ greatly, under different environmental conditions. A well-known example is that eggs of certain turtle species hatch male or female offspring depending on the temperature and thus depending on the environment. Butterflies produce different wing colors depending on the season. More daylight and lower temperature produce a dark type, less light produces an orange type.
Facilitated Variation Theory
The decoding of the genetic basis of the developmental processes in growth has shown that the essential processes are organized in development modules. Dozens to hundreds of genetically coded structures and structural units are controlled synchronously via shared regulatory units. "Master control genes" at key points can induce the development of entire organs, e.g. B. The pax6 gene can induce the development of functional eyes everywhere. The triggering regulatory units, mostly cellular signaling pathways and (controlled by transcription factors) cis-regulatory sections in the genome apart from the protein-coding sequences, by no means control development down to the last detail, but rather form switches that can switch on or off coordinated developmental pathways. The genetic basis of the control path is therefore different from that of the structure itself controlled by it. This means that it can vary and be selected independently of it. Sean Carroll coined the image of the “genetic toolbox”.
Other processes are not genetically determined down to the last detail. The development program only provides a largely shapeless basic structure, which is then only shaped in detail by the influences of the environment: for example, the maturation of the central nervous system, in which the countless synaptic connections between the nerve cells reinforce those required and those that are not used go. This means that the detailed architecture does not need to be genetically specified.
The authors Kirschner and Gerhart summarize the impact of these findings on the theory of evolution, they speak of facilitated variation ( facilitated variation ).
Conserved core processes
The basic structures of the cell organization and many of the structures on which the body plan and its organs are based are therefore regarded as conserved core processes. They then serve as raw material for fine control by the development modules. The individual processes do not change. Cell behaviors can therefore be combined in an evolutionary way or used in a new way. According to Kirschner and Gerhart, important examples of such conserved core processes are:
- the uniform genetic code of all living things
- the selectively permeable cell membrane for communication between cells as well
- the identical function of the Hox genes.
From the point of view of evolutionary developmental biology, the stable core processes allow manifestations or properties that allow easier phenotypic variation. According to Kirschner / Gerhart:
- exploratory processes,
- weak regulatory couplings and
- Compartment formation in the embryo.
Exploratory behavior
The differentiated formation of tendons , muscles , nerves and blood vessels during development is not given in detail by the genome. The way they originated can be described as exploratory . Cells show alternative reactions depending on their cellular environment. In this way, cells can create “branched structures” that optimally fill the desired tissue space (e.g. blood vessel, nervous system ) (Fig. 2). Exploratory structures are highly adaptable as they evolve.
Weak regulatory couplings between cells
The new combinations of the core processes necessary for evolutionary variation are mediated by cell communication. The authors speak of weak regulatory links here . "Weak", because the cell signal does not control the development in detail, but only switches it on, ie it has only a weak relationship to the specifics of the output in the target area. As a rule, the signal substance at the target address determines the “on” or “off” for the expression of a gene present there, this can be in the same cell or in a different cell. What exactly happens then, however, is determined by its own regulation in the target area. The cellular mechanism in the target area has been developed earlier and only needs to be activated for the specific response. An example of weak regulatory couplings are, for example, the process of the organism's insulin release, which is controlled in several stages by cells, after the supply of glucose, as well as many other metabolic processes.
Compartmentalization
It is only in the course of development that differentiated cells form for specific tissue types (skin, muscles, nerves, organs, etc.). There are regions of the embryo in which one or a few very specific genes of the cells are expressed and certain signal proteins are produced in a very specific phase of development. The ability to activate differently conserved core processes in different places in the organism and to actually create these reaction spaces is what they call compartmentalization . An insect embryo forms approx. 200 compartments in the middle phase of development. Compartment maps serve as a framework for the arrangement and construction of complex anatomical structures of living beings. Each animal tribe has its typical card. The expression of these compartments is the actual task of the hox genes.
The organisms, i.e. H. the phenotype, therefore, play a major role in determining the nature and degree of variation. Phenotypic variation cannot be arbitrary. Rather, facilitated variation causes an influenced “presorted” output of phenotypic variation by an organism. Variation is facilitated primarily because there is so much novelty available in what organisms already have.
Epigenetic inheritance
It has only gradually become clear since the 1980s that there are ways in which phenotypic features apart from DNA and the sequence of their base pairs can be inherited from one generation to the next. In contrast to genetics, these processes are called epigenetics . The proponents of the synthetic theory of evolution have dealt with such processes only reluctantly, because they had a " Lamarckist " overtone for them; H. reminded of the notions of the inheritance of acquired traits that had just been overcome. The extent and role of epigenetic inheritance are active research fields. In her extensive work on phenotypic evolution (2003), West-Eberhard was one of the first to provide numerous examples that prove that the environment can have a direct effect on development processes and in this way also on evolution. An extensive theory on the special role of epigenetic inheritance comes from Jablonka and Lamb.
Known mechanisms of epigenetic inheritance include (Fig. 3):
- DNA methylation . If bases of the DNA strand are enzymatically methylated, they are quasi “muted”. This means that individual genes or parts of the genome (e.g. the entire paternal or maternal genome) can be excluded from expression. Inheritance of methylation patterns is obviously more common in plants than in animals (plants do not have a germline ), but plays a role in both. Patterns of methylation play a major role both in controlling development and in responding to environmental stressors.
- Regulatory RNA. Parts of the genome do not code for proteins, but for (mostly short) RNA sequences that can play an important role in the control of gene expression.
- Histone complexes. Histones are proteins that accompany DNA and play a role in support and "packaging". In individual cases it has been shown that different modes of DNA-histone complexes can exist. DNA that is particularly “tightly” packed is transcribed less often.
Some authors want a wide range of other factors not inherited through DNA, including human culture, to be treated as epigenetic factors.
Important potential effects of epigenetically inherited processes include:
- Epigenetically determined traits are more labile and vary significantly more. The response norm of a population to environmental stress can therefore be considerably increased through epigenetic variants . The epigenetic variation may provide an additional field for short-term variation possibilities that can react faster than the mutation of the DNA.
- Epigenetically controlled trait manifestations provide a way by which the trait variation within a population can change quickly and synchronously in a certain direction when the environmental conditions change.
- It seems conceivable that within such epigenetically shaped populations a mutation occurs which fixes the phenotype generated by modifiers as a hereditary variation. As a result, advantageous mutations, which are necessarily very rare at the beginning, can have a decisive advantage and not their occurrence as such, but their fixation in the population are strongly promoted. This process is known as “ genetic assimilation ” (see also: Baldwin effect ).
Today there are already some empirical experiments that confirm genetic assimilation (Waddington 1953 with changes in the veins on fly wings, Nihjout 2006 with color variations in the tobacco hawk caterpillar). Due to the described existence of environmental factors, such design changes can initially be initiated. The system is capable of self-organization in order to react to such influences. However, it should be noted that the proposed mechanism is still largely speculative.
Development creates phenotypic innovation

According to the classical synthetic theory of evolution, phenotypic innovations, i.e. completely new structures in the body plan, cannot in principle be distinguished from variations. The most important mechanism for generating completely new structures is then the function change originally adaptively created structures for a different purpose (so-called pre-adaptations , also: exaptations), e.g. B. the spring originally created as insulation for the wing of the birds.
Some representatives of evolutionary developmental biology consider other, additional mechanisms to be necessary for innovations to occur.
Müller defines three types of innovations. From Evo-Devo's point of view, Novelty Type 2 is particularly important: A phenotypic innovation is a new construction element in a building plan that has neither a homologous equivalent in the previous species nor in the same organism (Fig. 4 and 5). A distinction is made between three phases in the development of such evolutionary innovations: initiation (mostly due to changed environmental conditions), realization (changes in the body plan made possible by the control in the development process) and accommodation.
Within the theory, it is assumed that new phenotypic elements are first epigenetically fixed and then assimilated . "The innovation feature must be accommodated in the already existing construction, development and genome system in order to ensure functionality and inheritance". "It seems to be the rule that epigenetic integration precedes genetic integration" or, as West-Eberhard puts it, "Genes are followers in evolution". "The genetic integration stabilizes and overdetermines the generative process (innovation process) and results in an ever closer mapping between genotype and phenotype".
Small causes (both environmental and mutation) can result in strong phenotypic changes. The self-organizing properties of the development system are responsible for such non-gradual, discontinuous reactions. An example of a spontaneous construction of the development system is a hand with six fingers ( polydactyly ) (Fig. 5).
The example shows that embryonic development is able to produce such phenotypic variation and that six fingers on one or both hands could represent a possible evolutionary variation. The development can create a fully integrated feature: nerves, muscles, joints, skeleton of the finger and its size are fully functional integrated into the anatomy of the hand.
Relationship between genetic and epigenetic dimensions
Building on progressive (comparative) genome sequencing, evolutionary developmental biology also examines the extensive gene regulation during development.
Sean B. Carroll or Wallace Arthur , but also Paul Layer accordingly see the gene regulation processes with changing combinations of gene switches as the predominant influencing factors on the development of the organism and on its potential for change. From this point of view, mutations in the regulatory genome are more essential for organismic evolution than mutations in structural genes.
Other researchers such as Marc Kirschner , Gerd B. Müller , Massimo Pigliucci or Mary Jane West-Eberhard go further and consider the entire developmental apparatus as a complex system that operates on the various genetic and epigenetic levels (DNA, cell nucleus, cells, proteins, cell communication, cell aggregates , Organism, environment) can act and react in a complex way with the presented mechanisms.
Directed variation in phenotypic characteristics
Directed development describes how the direction of evolutionary change is influenced by the non-random structure of variation. There are numerous examples of directed variation. For example, a group of millipedes with more than 1000 species shows only odd numbers of pairs of legs. The fact that these animals do not have an even number of pairs of legs is due to the mechanism of segmentation during embryonic development, which does not allow this. Skinks , a species-rich family of lizards, come in very different sizes. They have very short to no extremities. The toe reduction with increasing body size of different species takes place in exactly the opposite order as the formation of the toes in the embryonic development. The toe that is developed first in the embryo is also the first to disappear in the case of evolutionary toe reduction; the one that is developed last is the last. This is an example of a non-random, directed variation.
In the polydactyly form of the Hemingway mutant in Maine Coon cats, there are variable additional toe numbers. The variation is plastic . According to a current study of the polydactyl toe numbers of 375 Hemingway mutants, there is a directed developmental variation in the sense that the number of additional toes follows a discontinuous statistical distribution and is not randomly evenly distributed, as would be expected with the identical point mutation. The directionality is not a result of natural selection, since the phenotypes are considered at birth and natural selection has no point of attack at this point. Such a directionality in embryonic development is alien to the synthetic theory of evolution. At most, natural selection can bring about direction there.
The variation is a polyphenism . In the Hemingway mutant of the Maine Coon (wild type: 18 toes), polydactyly occurs in some cases with 18 toes by extending the first toe into a three-jointed thumb; Much more often, however, there are 20 toes and, with decreasing frequency, 22, 24 or 26 toes (Fig. 6), more rarely also odd toe combinations on the feet. The directionality of the toe numbers is the result of development mechanisms for the formation of the toes. While the underlying genetic mutation itself can be random, the phenotypic result, i.e. the statistical number of toes, is not random but directed (see Fig. 6). Another directionality is the difference in the number of toes on the front and rear feet. A slight left-right asymmetry in the number of toes can also be observed.
Methods of empirical research
Evolutionary developmental biology uses developmental and molecular biological laboratory methods in empirical research to identify factors and control mechanisms for the formation and evolutionary change of tissues, organs and morphological structures. The occurrence of such changes is reconstructed in the course of the tribal history of the organisms.
Initially, the focus was on experimental transplant experiments on embryos. For example, grafts have been removed from vertebrate extremities and replanted in other locations. More recently, in-situ hybridization and, above all, gene knockout have been used in molecular biology . By switching off genes, one can infer their function in development. One also speaks of gain of function or loss of function experiments. Imaging microCT methods (Fig. 7) and computer tomography in the micro and nanometer range make an important contribution to Evo-Devo. With contrast media, gene activities can be made visible, so that the contribution of one or more genes to the spatio-temporal development process can be observed. In addition to the gene expression level, what is needed is the “calibrated, three-dimensional representation of anatomical structures in their natural appearance and spatial relationships, as close to the natural state as possible for prepared specimens” (Metscher).
The BDTNP (Berkeley Drosophila Transcription Network Project) has a comprehensive claim to map the complete embryonic course of Drosophila with imaging methods. The aim is to create complete gene expression atlases. A data set of 75,000 images per embryo is generated with activities of around 50% of the genes made visible. This "represents a solid observational basis for analyzing the relationship between gene sequence, tissue-specific gene expression and development in the animal world" (Tomancak). The complete atlas contains the data of all transcription products of the Drosophila genome in all phases of development. In the future, this will lead to the “automated creation and storage of expression patterns of living species in four dimensions”. The BDTNP project shows the computer-aided statistical evaluation of specific gene expressions of hundreds of embryonic comparisons of Drosophila ( virtual embryos ) on the Internet with video streams today . The comparison of the processes serves to generate statistical probabilities for the development of phenotypic ranges of certain tissues. With stressors (heat, cold, nutrition etc.), expression patterns can be changed in the future, statistically evaluated and in this way possible evolutionary development paths can be identified.
Selected empirical research results
Receipts at the fruit fly
The British Conrad Hal Waddington (1942) had already found out that environmental factors affect heredity and evolution . Later (1953) he was able to empirically prove his theory on the basis of changes in the veins of the fruit fly ( Drosophila melanogaster ) by subjecting the fly eggs to short heat shocks for several generations. After a few generations, the cross struts on the wings were missing. If the heat shocks were removed as an external stressor in subsequent generations, the variations they induced in the wing vein system remained, that is, the cross bracing did not reappear. The heat shocks were a sufficiently strong impetus that previous development paths were abandoned. Masked paths that had already been created but had not been used up to that point came to light due to external influences. Development has been channeled into a new path, in Waddington's words . The environmental factor was only necessary until the newly channeled course of development, as Waddington put it, was subsequently also genetically assimilated . Here it remains channeled or stable again, even with new mutations occurring, until either a mutation or new environmental influences are strong enough that the channeling reaches its limits. If necessary, this then leads to a new variation of the phenotype under the influence of threshold value effects , as explained above.
What Waddington could not show with the laboratory tests is how an adaptive way is created that reacts appropriately to an environmental factor (here: heat). The variation of the wing veins is not an adaptive feature to heat exposure. "It is by no means certain that he would have encountered any particular adaptive morphology with any appreciable frequency".
Change in beaks in Darwin finch species
Sean B. Carroll was able to make changes to the patterns of butterfly wings in the laboratory. By determining the corresponding gene switch combinations in the development of the butterfly, it was possible to vary the patterns on the wings. In 2007, Peter and Rosemary Grant demonstrated in Darwin finch species on the Galápagos Islands that the beaks were transformed in just a few generations due to changes in the food supply (initiator).
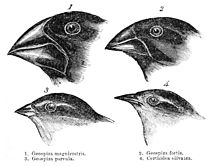
In this context, it was possible to identify a growth factor protein that is significantly involved in beak formation in the embryo, and it was also possible to show that this protein is formed to different degrees or with different lengths of correlation in different beak shapes. Kirschner / Gerhart also mention that the said protein (it's called BMP4 and is produced in neural crest cells ) was experimentally implanted in the neural crest of a chicken, where the shape of the beak also changed. The chicken developed wider and larger beaks than normal. Other growth factors do not have this effect. So although the experimentally manipulated beak changes its size or shape, it is still integrated into the anatomy of the bird's head. “There is no monstrous undesirable development” (Kirschner / Gerhart).
Beak formation is a complex developmental process that involves five nests of neural cells. The nests receive signals from facial cells in the five locations and respond to them. Therefore, features that affect the neural crest cells influence beak growth in a coordinated manner. Using this and other examples, the prevailing synthetic theory of evolution should be able to plausibly explain how, in just a few generations, just through the interplay of random mutation and selection, such an extensive, coordinated phenotypic variation can arise that requires a mutual interaction of many separate development parameters.
Evo-Devo shows the explained mode of action with this example: Small cause (one or a few quantitative, regulatory protein changes, caused) leads to large effects (integrated change in the shape of the beak), controlled by epigenetic processes of development, in particular by a broad adaptive cell behavior of the Neural crest cells of the beak and the visual area. From the well-researched knowledge of the development of the beak and its modifications it can be concluded that “quite extensive changes in beak size and shape can be achieved with a few regulatory mutations rather than with a summation of long sequences of small changes”. In this example, it has not been finally researched what triggers the changes in the Bmp4 level in development. One possibility are genetic random mutations, more likely are developmental reaction pathways to the stress of the animals, which results from the constant change in the food supply. This change was documented by the grants in connection with the variation of the beaks.
Belyayev's taming of silver foxes
The attempt by the Russian geneticist Dmitri Konstantinowitsch Beljajew , whose team selected silver foxes for tame over a period of 40 years, has become known . One wanted to find out what consequences the selection for tameness (or against aggression) has. Belyayev's hypothesis was that diverse morphological, physiological and behavioral changes could occur, similar to what was observed in the domestication of wolves to dogs over 10,000 years ago. For the experiment, particularly tame-looking foxes from fur farms were selected, a breed line established and then selected for tameness (and only for that, not for other morphological or physiological changes).
Belyayev's team took great care to ensure that mechanisms such as inbreeding or polygeny did not influence the results. Nevertheless, from the fourth generation onwards, in addition to behavioral changes, typical pet features such as white fur markings, hanging ears, short legs and snouts also appeared in the foxes. Beljajew was able to reproduce this in the core by means of parallel experiments on other species (otters, rats).
As the project progressed, changes in the neurochemical and neurohormonal mechanisms that play an essential role in these areas have been revealed, mainly cortisol , adrenaline and serotonin levels. The greatly reduced level of the "stress hormones" cortisol and adrenaline and the reduced serotonin level associated with aggressiveness are plausible explanations for the increasing tameness. In addition to the behavioral changes, these reactions in the hormone levels may also have contributed to the developmental changes in Belyayev's experiment. One plausible model assumes that a delay in the innate fear response in young animals leads to increased familiarity with humans. This developmental delay is possibly coupled with other developmental delays through developmental constraints. The typical anatomical effects make the animal more puppy-like ("neoteny"), often coupled with increased fertility. Trut particularly pointed out that the fertility cycle in female wild animals is normally genetically “hardwired” and, even in decades of direct breeding attempts, could not be changed in the past in the interests of the breeders. Such changes are achieved in Belyayev's experiment as a by-product, and on the way exclusively via a specific behavioral selection, there were a number of coordinated changes in the female cycle (about 1 month earlier onset of fertility). Trut points out that pregnancies also occurred outside of normal gestation periods, but none of the boys born in seasonal cycles that deviated from the natural cycle survived. A reason for this could not be found.
The changes observed show a surprisingly rapid change to the pet, with most of the traits occurring unintentionally, as a pure by-product of the selection for tameness. The change is many times too fast for a conventional genetic explanation of the accumulation of point mutations. A change in the gene expression of (previously unidentified) master genes with a development-controlling effect can be assumed. A purely epigenetic mechanism via “silencing” of a development gene through DNA methylation of a development gene or its regulatory sequences, which appears possible due to hormonal influences, would be possible.
Evolution of the eye
By analyzing spontaneous mutations in the fruit fly Drosophila , which lack eyes, geneticists have succeeded in identifying a key gene from the regulatory cascade of eye development. This gene turned out to be a transcription factor , that is, it codes for a protein which binds to the DNA and thereby increases or prevents the transcription of other genes. The gene called pax6 belongs to a whole family of regulatory genes that control all developmental processes. In a sensational experiment, the researchers succeeded in creating (functional!) Eyes in other parts of the body through artificially induced expression of the gene: on the antennae, on the base of the wings, on the thorax, etc. Through almost routine comparisons with the genome of other organisms today It turned out that genes with a similar sequence, which in all probability are homologous , were found in animal species from almost all of the animal strains examined : z. B. in vertebrates (mouse, human), molluscs (mussels, squids), nematodes and the like. v. a., and in all cases (among a few other tasks) it was involved in eye development. Even the primitive eyespots of the flatworm Dugesia and the lens eyes on the edge of the umbrella of the box jellyfish Tripedalia cystophora were controlled by the same or a homologous gene.
This was unexpected because these animals have diverged in evolution at least since the Cambrian more than 540 million years ago. Nevertheless it was possible to induce eyes in the fruit flies with the mouse gene. Eye development requires the finely tuned interaction of a few hundred effector genes.
This can best be explained by the fact that these genes, which in the jargon of geneticists are “downstream” of pax6, contain binding sites (so-called cis-regulatory elements ) for the Pax6 protein. Pax6 is only one factor in a finely balanced network of signal chains and control paths, which is hardly known in detail. Sean Carroll coined the expression depth homology for such development paths that have been preserved in evolution over hundreds of millions of years .
When looking at the eyes in detail, however, it becomes apparent that it is not necessarily likely that a simple further development of an eye that has already been created makes up the whole story. All eyes of all animals have the same light-sensitive molecule, a variant of the visual pigment rhodopsin (which is already found in unicellular organisms and also in prokaryotes). In addition to the visual pigment, even the simplest eyes include a light-shielding pigment (for directional vision), except for the simplest constructions, a translucent "glass body" made of a transparent protein (called "crystalline"). A comparison of different eye types shows that different organisms use different pigments (melanin, pterin, ommochrome) and, above all, completely different crystallins. In addition, almost all crystallins are enzymes or derivatives of those that have completely different, essential tasks to perform elsewhere in the body. In addition, there are two versions of the receptor cells, as “rhabdomeric” and “ciliary” receptors with completely different cell structures. The rhabdomerische version is found in the arthropods, the ciliare in the vertebrates, but also in the box jellyfish. The essential components of the eye are therefore under development control that has been highly conserved in evolution, but underneath them are almost randomly “jumbled up”. This seems most likely to be explained by the fact that in the construction of the increasingly complex eye, structures that were originally independent and for a different purpose, but also development paths and signaling pathways, were used in addition to their original function in eye development. This can best be explained by the fact that they have evolved cis-regulatory sequences that have been acquired through control genes of eye development such as e.g. B. pax6 are controllable (the genes are identical in all body cells!). The overall development path is thus homologous, but the other structures such as pigments, vitreous bodies, lenses, etc. are probably convergent formations. Neither new protein families nor even new genes were invented for their creation, but existing ones were repurposed (“recruited” or “co-opted”).

Changes in the skeleton during the formation of the turtle shell
The shell of the turtle is an anatomically extremely complex formation, in which both bones (especially the ribs) and skin ossification (osteoderm) are involved. More recently, this structure, which has been puzzling since the anatomical studies of the 19th century, has again attracted a lot of attention from evolutionary biologists, because an influential theory postulated that the developmental facts that have become known are that the armor is formed by macromutation (also known as saltation ) suggest and thereby partially refute the synthetic theory of evolution. However, it has been shown that a plausible explanation is also possible without saltation. In addition to more recent developmental genetic findings, an important reason was that fossils of turtles with incomplete armor were recently discovered for the first time.
The development of the shell can therefore be imagined as follows: The decisive morphological feature is the development of the ribs, which do not more or less enclose the chest cavity as in all other vertebrates, but which grow into the upper (anatomically: dorsal) body wall. Here they combine with skin ossification, which presumably developed independently (corresponding formations are widespread in vertebrates). In embryonic development, both formations are controlled via a cellular signal path (the so-called wnt path), which otherwise z. B. is involved in the formation of limbs. A corresponding partial remodeling of already existing developmental pathways (and their genes) is referred to as "cooptation" and obviously occurs more frequently, even if the details are not completely clear. The most likely explanation is the conversion of “genetic switches” (so-called cis-regulatory sequences), through which the expression of a gene in a new functional context is possible without the gene itself changing. In the course of this redesign, the lateral body wall of the turtle embryo folded inwards. As a result, the shoulder girdle, which otherwise lies above the ribs, was shifted inward (the original positional relationship can still be read from the muscles attached to it). The belly armor (the plastron) was created in parallel in a not yet fully understood way. A cellular signaling pathway that corresponds to the one that leads to ossification of the cranial bones is presumably involved in its development. Interestingly, a 220-million-year-old aquatic turtle fossil discovered in China had a complete belly armor, while a back armor was missing.
Although not all details have been clarified yet, there is a convincing model of the gradual development for an initially puzzling structure such as the turtle shell. This clearly shows how intensive cooperation between all disciplines (here: paleontology, embryology, developmental genetics) can gradually unravel problems that initially seem unsolvable.
Flösselike: Experiment on shore leave

In an eight-month trial with juvenile pikeperch of the genus Polypterus from tropical Africa ( Polypterus senegalus ), it was determined for the first time in 2014 how well pikeperch adapt to the conditions on land if they are completely deprived of their aquatic way of life. It turned out that the animals were able to adapt surprisingly quickly to the new conditions. The test animals not only survived, but even flourished in the new environment. Their adaptations included changes in both the muscles and the bone structure. The test individuals were able to walk significantly better on dry land than the aquatic control animals. For evolutionary biologists in evolutionary developmental biology, this unexpectedly high developmental plasticity allows conclusions to be drawn about how the first sea creatures, such as the Tiktaalik , landed 400 million years ago and how tetrapods gradually formed with the transition from fins to extremities . This experiment with Flösselhechten confirmed for an evolutionarily very important systemic transformation of shore leave , finally emerged all land vertebrates from the hypothesis that animals can adapt both their anatomy and their behavior in response to environmental changes plastically in evolutionary short time. In the long term, genetic mutations could support the conditions created by the new environmental situation and ensure suitable inheritance. The evolutionary sequence would therefore not be genetic mutation, natural selection, adaptation in the population, but the other way round: change in environmental conditions, permanent, not yet genetically hereditary phenotypic adaptation, supporting genetic mutations.
Evo-Devo also provides an explanation for the elimination of legs in the evolutionary transition from lizards to snakes . According to Paul Layer, this required the inhibition of expression of only 2 Hox genes (c6 and c8), as has been proven empirically.
Consequences for the theory of evolution
Evo-Devo explains the evolution of organismic form with the causal-mechanistic changes of the overall system development (consisting of the semi-autonomous subsystems genome, cells, cell associations, organism) and the effect of environmental influences within the framework of the extended synthesis in the evolution theory . In contrast, the standard evolutionary theory is more statistical-descriptive, as it does not take into account factors inherent in the organism for the development of variation and innovation. Rather, it leaves the evolutionary process “exclusively” to chance mutation and externalistic natural selection. - In any case, if systematic variations in the reproduction of living beings through sexual reproduction are ignored (variations in the synthetic theory of evolution refer not to individual organisms but to populations. In sexually reproducing species, evolutionary variations therefore occur systematically .). From a classical point of view, evolution thus takes direction through the interaction of (sexually reproducing species systematized ) variation and selection. Hence the extraordinary evolutionary stability of the prokaryotes becomes understandable. This is in contrast to the evolutionary “instability” of sexually reproducing species (cf. Cambrian Explosion ).
The importance of chance in generating morphological variation is being reassessed by evolutionary developmental biology. There are explainable, predictable, regular laws of the development processes which strongly influence the (essentially still random) variation. This is one of the main differences between evo-devo and synthetic evolution theory. Natural selection continues to make its contribution ( survival of the fittest ), but its explanatory value for the emergence of organismic form and complexity is relativized and compared with the explanatory value of construction . Selection remains a basic condition of evolution. But it can only "decide digitally" what the development tells it to do.
Evo-Devo criticizes the reductionist thinking that some authors call “gene-centric” and the reductionist thinking that was especially promoted by Richard Dawkins . For researchers such as Gilbert, Kirschner, Müller, Pigliucci, West-Eberhard and others, genes are neither the exclusive nor the main addressee of natural selection. A strictly deterministic relationship between genotypic and phenotypic evolution is also not assumed. In connection with the evolution of organismic form, the epigenetic development processes are considered indispensable for the generation of the phenotype and the generation of morphological variation and innovation. This does not remove the paramount importance of genes, but does relativize them to a certain point.
The theory of evolution is thus in a post-genocentric era (Müller-Wille, Staffan and Rheinberger ), in which it takes up the complex, recursive, epigenetic relationships and has to integrate them into a congruent theoretical framework . As an example, the Altenberg-16 group presented a new interdisciplinary approach with the concept of "extended synthesis" published in 2010 and expanded upon in the following.
New systemic view of development
Some researchers, among them Brian K. Hall, Rudy Raff, Gerd B. Müller , Walter Gilbert , Marc Kirschner , Massimo Pigliucci , Mary Jane West-Eberhard , go beyond the analysis of gene regulation and extend the investigation to the entire system development as a complex genetically / epigenetically evolved apparatus that interacts with the environment as an integrated, multi-causal, non-linear, open system.
Causal explanations for the development and variation of organismic forms are not only sought by these researchers on the gene / gene regulation level, but also or primarily in the epigenetic properties of cells, cell aggregates, self-organization, organism and environment (see Fig. 5).
Evo-Devo does not use epigenesis synonymously with epigenetics ; H. it does not refer to gene regulatory aspects such as DNA methylation , but the sum of the non-programmed factors in embryonic development, such as B. chemical, physical or dynamic factors that influence the organizational development process.
Eco-Evo-Devo
The consideration of the environment significantly expands the discipline (see Fig. 1).
In this context, one also speaks of Eco-Evo-Devo . Leigh Van Valen defined evolution in 1973 as "the control of development by the environment".
This field includes considerations of phenotypic plasticity or developmental plasticity; H. the ability of the organism to develop different phenotypes depending on changing environmental conditions. In addition to the CH Waddington already cited, the book by Mary Jane West-Eberhard Development Plasticity and Evolution (2003) and the interdisciplinary book by Scott F. Gilbert and David Epel Ecological Developmental Biology (2009) should be mentioned here. The inclusion of the environment and thus the ability of evolution to interact with the environment on the way through development is becoming a growing research topic (see The Evo-Devo Research Topics ).
literature
Conceptual basics
- Ron Amundson: The Changing Role of the Embryo in Evolutionary Thought. 2005, ISBN 0-521-80699-2 .
- Wallace Arthur: Biased Embryos and Evolution. Cambridge University Press, 2004, ISBN 0-521-54161-1 .
- Ingo Brigandt: Beyond Neo-Darwinism? Recent developments in evolutionary biology. In: Philipp Sarasin , Marianne Sommer: Evolution - An interdisciplinary manual. JB Metzler, 2010, pp. 115-126.
- Sean B. Carroll: EvoDevo - The new picture of evolution. Berlin 2008, ISBN 978-3-940432-15-5 . (Orig .: Endless Forms Most Beautiful, USA 2006)
- Scott F. Gilbert: The morphogenesis of evolutionary development biology. 2003.
- Mark C. Kirschner, John C. Gerhart: The Solution to Darwin's Dilemma - How Evolution Creates Complex Life. Rowohlt, 2007, ISBN 978-3-499-62237-3 . (Orig .: The Plausibility of Life (2005))
- Alessandro Minelli, Giuseppe Fusco (eds.): Evolving pathways - key themes in evolutionary developmental biology. Cambridge University Press, Cambridge / New York 2008.
- Alessandro Minelli: Forms of Becoming - The Evolutionary Biology of Development. Princeton University Press, 2009, ISBN 978-0-691-13568-7 .
- Gerd B. Müller, Stuart A. Newman: Origination of Organizmal Form - Beyond the Gene in Development and Evolutionary Biology. MIT-Press, 2003, ISBN 0-262-13419-5 .
- Gerd B. Müller: Evodevo as a discipline in Minelli & Fusco. 2008.
- Christiane Nüsslein-Volhard : The Becoming of Life - How Genes Control Development. dtv, 2006, ISBN 3-423-34320-6 .
- Massimo Pigliucci, Gerd Müller: Evolution - the Extended Synthesis. MIT Press, 2010, ISBN 978-0-262-51367-8 .
- Andreas Sentker: Darwin's wise heirs. In: The time. No. 40, September 29, 2005.
- Mary Jane West-Eberhard: Development Plasticity and Evolution. Oxford University Press, 2003.
Further reading and internet articles
- BDTNP Berkeley Transcription Drosophila Network Project
- Scott F. Gilbert, David Epel: Ecological Development Biology. Integrating Epigenetics, Medicine and Evolution. Sinauer Ass. USA, 2009.
- Eva Jablonka, Marion J. Lamb: Evolution in four dimensions. Genetic, Epigenetic, Behavioral and Symbolic Variation in the History of Lfe. MIT Press, 2005. (PDF; 6.2 MB)
- Gerd B. Müller, Stuart A. Newman: The Innovation Triad. On EvoDevo Agenda. In: Journal of Experimental Zoology. 304B, 2005, pp. 487-503.
- M. Neukamm: Evolutionary Developmental Biology: New Paradigm. In: Laborjournal. 15 (11), 2009, pp. 24-27. (pdf)
- Frederic Nihjout: Researchers evolve a complex genetic trait in the labratory. 2006.
- Massimo Pigliucci: What, if anything, Is an Evolutionary Novelty? In: Philosophy of Science. 75, 12/2008, pp. 887-898.
- Pavel Tomancak et al .: Patterns of gene expression in animal development. 2007.
- Lyudmila N. Trut: Early Canid Domestication: The Farm-Fox Experiment. In: American Scientist. Volume 87, 1999.
- Emma Young: Rewriting Darwin. The new non-genetic inheritance. In: New Scientist magazine. 2008.
Individual evidence
- ↑ Ingo Brigandt: Beyond Neo-Darwinism? New developments in evolutionary biology, chap. Evolutionary developmental biology in: Philip Sarasin and Marianne Sommer (eds.). Evolution. An interdisciplinary manual. JB Metzler 2010
- ↑ For a comprehensive overview of the role of the embryo in the history of evolutionary theory, see Ronald Amundson: The Changing Role of the Embryo in Evolutionary Thought. Roots of Evo-Devo. Cambridge University Press, 2005.
- ^ Brian K. Hall: Balfour, Garstang and de Beer: The First Century of Evolutionary Embryology. In: American Zoologist. 40 (5), 2000, pp. 718-728.
- ^ Alan C. Love, Rudolf A. Raff: Knowing your ancestors: themes in the history of evo-devo. In: Evolution and Development. 5 (4), 2003, pp. 327-330.
- ^ Scott F. Gilbert: The morphogenesis of evolutionary development biology. 2003, p. 471.
- ^ Eva Jablonka, Marion J. Lamb: Evolution in four dimensions. Genetic, Epigenetic, Behavioral and Symbolic Variation in the History of Life. MIT Press, 2005, pp. 261-266.
- ^ Scott F. Gilbert: The morphogenesis of evolutionary development biology. 2003, p. 474.
- ↑ M. Pigliucci, G. Müller: Evolution. The Extended Synthesis. 2010, chap. 1: Elements of an Extended Evolutionary Synthesis.
- ↑ M. Pigliucci, G. Müller: Evolution. The Extended Synthesis. 2010, chap. 1: Elements of an Extended Evolutionary Synthesis.
- ↑ M. Pigliucci, G. Müller: Evolution. The Extended Synthesis. 2010, p. 13.
- ^ Mary Jane West-Eberhard: Development Plasticity and Evolution. 2003, p. 6 ff.
- ^ Scott F. Gilbert: The morphogenesis of evolutionary development biology. 2003, p. 470.
- ^ A b Scott F. Gilbert: The morphogenesis of evolutionary development biology. 2003, p. 473.
- ↑ Master control genes or master regulator genes are genes "which continuously express one or more transcription factors in certain differentiating cells" (Kirschner and Gerhard 2005, p. 384)
- ↑ Christiane Nüsslein-Volhard: The Becoming of Life - How Genes Control Development. 2006, p. 93.
- ↑ Paul G Layer: Evo-Devo: The molecular developmental biology as a key to understanding the theory of evolution. In: Journal of Pedagogy and Theology. 61 (4), 2009, p. 328.
- ^ Gerd B. Müller, Stuart A. Newman: The Innovation Triad. On Evo-Devo Agenda. In: Journal of Experimental Zoology. 304B, 2005, pp. 387-503.
- ↑ Mark C. Kirschner, John C. Gerhart: The Solution to Darwin's Dilemma - How Evolution Creates Complex Life. Rowohlt, 2007 (Orig .: The Plausibility of Life. 2005)
- ↑ M. Pigliucci, G. Müller: Evolution - the Extended Synthesis. MIT Press, 2010, chap. 10 u. 12
- ^ Mary Jane West-Eberhard: Development Plasticity and Evolution. Oxford University Press, 2003.
- ^ Mary Jane West-Eberhard: Development Plasticity and Evolution. 2003, p. 7 with reference to Buss 1987.
- ↑ A. Minelli, G. Fusco (Ed.): Evolving Pathways - Key Themes. In: Evolutionary Developmental Biology. 2008, p. 2.
- ^ Gerd B. Müller: Extending the Evolutionary Synthesis. 2009.
- ↑ Sean B. Caroll: Evo-Devo - The new picture of evolution. Berlin 2008. (Orig .: Endless Forms Most Beautiful. USA 2006)
- ↑ Gerd B. Müller: Evo-devo: extending the evolutionary synthesis. In: Nature Reviews Genetics . 2007, p. 2.
- ^ Scott F. Gilbert: The reactive Genome. In: Gerd B. Müller, Stuart A. Newman: Origination of Organizmal Form. Beyond the Gene in Development and Evolutionary Biology. 2003, pp. 87-101 f.
- ^ Scott F. Gilbert: The reactive Genome. 2003, p. 93 f.
- ↑ Hans Meinhardt: The simulation of embryonic development. In: Spectrum of Science. 03/2010.
- ↑ MC Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 264.
- ↑ MC Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 302.
- ↑ a b M. C. Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 382.
- ↑ MC Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 328.
- ↑ MC Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 333.
- ↑ MC Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 369.
- ↑ Christina L. Richards, Oliver Bossdorf, Massimo Pigliucci: What role does heritable epigenetic variation play in phenotypic evolution? In: BioScience. 60 (3), 2010, pp. 232-237.
- ↑ Ryan A. Rapp, Jonathan F. Wendel: Epigenetics and plant evolution. In: New Phytologist. 168, 2005, pp. 81-91.
- ^ Eva Jablonka, Marion Lamb: Evolution in Four Dimensions: Genetic, Epigenetic, Behavioral, and Symbolic Variation in the History of Life. MIT, 2005.
- ↑ E. Jablonka, G. Raz: Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. In: Quarterly Review of Biology. 84, 2009, pp. 131-176.
- ↑ EJ Finnegan: Epialleles-a source of random variation in times of stress. In: Current Opinion in Plant Biology. 5, 2002, pp. 101-106.
- ^ MJ West-Eberhard: Developmental Plasticity and Evolution. Oxford University Press, 2003.
- ↑ M. Pigliucci, G. Müller: Evolution - the Extended Synthesis. 2010 and there: G. Müller Chapter 12 Epigenetic Innovation p. 311.
- ^ Massimo Pigliucci: What, if anything, Is an Evolutionary Novelty? In: Philosophy of Science. 75, Dec 2008, pp. 887-898.
- ^ A b Gerd B. Müller, Stuart A. Newman: The Innovation Triad. On Evo-Devo Agenda. In: Journal of Experimental Zoology. 304B, 2005, pp. 487-503.
- ↑ M. Pigliucci, G. Müller: Evolution - the Extended Synthesis. 2010 and there: G. Müller chap. 12: Epigenetic Innovation. P. 314 ff.
- ↑ a b c Gerd B. Müller, Stuart A. Newman: The Innovation Triad. On Evo-Devo Agenda. 2005, p. 494.
- ^ MJ West-Eberhard: Development Plasticity and Evolution. Oxford University Press, 2003, p. 157.
- ↑ M. Pigliucci, G. Müller: Evolution - the Extended Synthesis. u. there G. Müller chap. 12: Epigenetic Innovation. 2010, p. 320 f.
- ↑ Sean B Carroll: Evo-Devo - The new picture of evolution. Berlin 2008. (Orig .: Endless Forms Most Beautiful. USA 2006)
- ^ Wallace Arthur: Biased Embryos and Evolution. Cambridge University Press, 2004.
- ^ Paul G Layer: EvoDevo: Molocular developmental biology as a key to understanding the theory of evolution. 2009, p. 324f.
- ↑ Complex means here in the sense of complexity research : interdependent (genome - development - evolution - environment), multi-causal, non-linear, open, etc. ( systems theory )
- ↑ Ronald A. Jenner: Evo-devo's identity: from model organism to developmental types. In: Alessandro Minelli, Giuseppe Fusco: Evolving Pathways. Cambridge University Press, 2008, p. 108.
- ^ W. Arthur: Biased Embryos and Evolution. Cambridge Univ. Press, 2004.
- ^ AE Greer: Limb reduction in the Scincid lizard genus Lerista 2nd Variation in the bone complements of the front an rear limb and the number of postsacral vertebrale. In: Journal of Herpetology. 24, 1990, pp. 142-150.
- ↑ a b Axel Lange, Hans L. Nemeschkal, Gerd B. Müller: Biased polyphenism in polydactylous cats carrying a single point mutation: The Hemingway Model for digit novelty. In: Evolutionary Biology. Dec 2013.
- ↑ Note: According to Metscher (Metscher 2009), the best devices today have a resolution of 60 nanometers. 1 nm = 1 billionth of a m or 1 millionth of a mm (e.g. a human cell has an average size of 10 to 20 micrometers, which is around 1000 larger.)
- ↑ a b c Pavel Tomancak et al: Patterns of gene expression in animal development. (Short version of the article: Global analysis of patterns of gene expression during Drosophila embryogenesis. In: Genome Biol. 8, 2007, no 145.1–145.34.
- ↑ Brian Metscher: microCT for comparative morphology: simple staining methods allow high contrast 3D imaging of various non-mineralized animal tissues. 2009. (online)
- ^ Conrad Hal Waddington: Genetic Assimilation of an Acquired Character. In: evolution. Vol. 7, No. 2, 1953, pp. 118-126.
- ↑ MC Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 316.
- ↑ Sean B. Caroll: Evo-Devo. 2008, pp. 192-209.
- ↑ The Darwin's Finches - Evolution in Time Lapse. Findings of a British couple from more than 30 years of research in the Galapagos Islands. In: Neue Zürcher Zeitung. July 12, 2006.
- ↑ Mark C. Kirschner, John C. Gerhart: The solution to Darwin's dilemma. 2007, p. 321f.
- ↑ a b M. C. Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 3121 with reference to studies by Clifford Tabin on Darwin's finches
- ↑ MC Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 318.
- ↑ a b M. C. Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 319.
- ↑ MC Kirschner, JC Gerhart: The solution to Darwin's dilemma. 2007, p. 320ff.
- ↑ The Darwin's Finches - Evolution in Time Lapse. In: Neue Zürcher Zeitung. July 12, 2006; P. u. R. Grant see backcrossing (introgression) as an adaptive cause for the variation of the beaks p. 3.
- ↑ Lyudmila N. Trut: Early Canid Domestication: The Farm-Fox Experiment. In: American Scientist. Vol 87, 1999.
- ↑ Lyudmila N. Trut: Early Canid Domestication: The Farm-Fox Experiment. In: American Scientist. Vol 87 (2), 1999, pp. 165f.
- ↑ Lyudmila Trut, Irina Oskina, Anastasiya Kharlamova: Animal evolution during domestication: the domesticated fox as a model. In: Bioessays. 31 (3), 2009, pp. 349-360.
- ↑ Lyudmila N. Trut: Early Canid Domestication: The Farm-Fox Experiment. In: American Scientist. Vol 87, 1999, p. 167.
- ↑ Georg Halder, Pstrick Call Aerts, Walter J. Gehring: Induction of ectopic eyes by targetes expression of the gene in Drosophila eyless. In: Science. 267, 1995, pp. 1788-1792.
- ↑ Walter J. Gehring, Kazuho Ikeo: Pax6: mastering eye morphogenesis and eye evolution. In: Trends in Genetics . 15 (9), 1999, pp. 371-377.
- ↑ Neil Shubin, Cliff Tabin , Sean Carroll: Deep homology and the origins of evolutionary novelty. In: Nature. 457, 2009, pp. 818-823.
- ↑ Joram Piatigorsky, G. Wisow: The recruitment of crystallins: new functions precede gene duplication. In: Science. 252, 1991, pp. 1078-1079.
- ↑ Pavel Vopalensky, Zbynek Kozmik: Eye evolution: common use and independent recruitment of genetic components. In: Philosophical Transactions of the Royal Society. B 364, 2009, pp. 2819-2832.
- ↑ Joram Piatigorsky: A Genetic Perspective on Eye Evolution: Gene Sharing, Convergence and Parallelism. In: Evolution: Education and Outreach. 1 (4), 2008, pp. 403-414.
- ^ SF Gilbert, GA Loredo, A. Brukman, AC Burke: Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. In: Evolution and Development. 3, 2001, pp. 47-58.
- ↑ Olivier Rieppel: Turtles as hopeful monsters. In: BioEssays. 23 (11), 2001, pp. 987-991.
- ↑ Chun Li, Xiao-Chun Wu, Olivier Rieppel, Li-Ting Wang, Li-Jun Zhao: An ancestral turtle from the Late Triassic of southwestern China. In: Nature. 456 (27), 2008, pp. 497-501.
- ^ Walter G. Joyce, Spencer G. Lucas, Torsten M. Scheyer, Andrew B. Heckert, Adrian P. Hunt: A thin-shelled reptile from the Late Triassic of North America and the origin of the turtle shell. In: Proccedings of the Royal Society. B 276, 2009, pp. 507-513.
- ↑ An overview in: Matthew K. Vickaryous, Jean-Yves Sire: The integumentary skeleton of tetrapods: origin, evolution, and development. In: Journal of Anatomy . 214, 2009, pp. 441-464.
- ↑ Shigehiro Kuraku, Ryo Usuda, Shigeru Kuratani: Comprehensive survey of carapacial ridge-specific genes in turtle implies co-option of some regulatory genes in carapace evolution. In: Evolution and Development. 7 (1), 2005, pp. 3-17.
- ↑ Shigeru Kuratani, Shigehiro Kuraku, Hiroshi Nagashima: Evolutionary developmental perspective for the origin of turtles: the folding theory for the shell based on the developmental nature of the carapacial ridge. In: Evolution and Development. 13 (1), 2011, pp. 1-14.
- ↑ Scott F. Gilbert, Gunes Bender, Erin Betters, Melinda Yin, Judith A. Cebra-Thomas: The contribution of neural crest cells to the nuchal bone and plastron of the turtle shell. In: Integrative and Comparative Biology. 47 (3), 2007, pp. 401-408.
- ^ Emily M. Standen, Trina Y. Du, Hans CE Larsson: Developmental plasticity and the origin of tetrapods. In: Nature. 513, September 4, 2014, pp. 54–58.
- ^ How fish can learn to walk. (Video)
- ^ Paul G Layer: EvoDevo: Molecular developmental biology as a key to understanding the theory of evolution. 2009, p. 329.
- ↑ John Dupré: Darwin's Legacy. The importance of evolution for the present of man. Suhrkamp, 2009 and there especially chap. The genocentric fallacy. P. 89ff.
- ↑ S. Müller-Wille, H.-J. Rheinberger: The gene in the age of post-genomics. A scientific inventory. Suhrkamp, 2009.
- ↑ John Dupré: Darwin's Legacy. Suhrkamp 2009 and There especially chapter: The genocentric fallacy. P. 89ff.
- ↑ M. Pigliucci, G. Müller (Ed.): Evolution. The Extended Synthesis. 2010.
- ↑ Kevin N. Laland, Tobias Uller, Marcus W. Feldman, Kim Sterelny, Gerd B. Müller, Armin Moczek, Eva Jablonka, John Odling-Smee: The extended evolutionary synthesis: its structure, assumptions and predictions. Proceedings of the Royal Society B: Biological Sciences 282 (1813). August 2015 DOI: 10.1098 / rspb.2015.1019 .








