Gentisic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Gentisic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 6 O 4 | ||||||||||||||||||
| Brief description |
light yellow, odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 154.12 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
199–200 ° C (decomposition) |
||||||||||||||||||
| pK s value |
2.95 (COOH) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Gentisic acid (2,5-dihydroxybenzoic acid) is an aromatic compound which is formally derived from both benzoic acid and hydroquinone (1,4-dihydroxybenzene). The structure consists of a benzene ring with one carboxy group (-COOH) and two hydroxyl groups (-OH) as substituents . It belongs to the group of dihydroxybenzoic acids and occurs in some plants such as caraway and lavender as well as gentian (scientific name Gentiana ).
Occurrence
Gentisic acid is a by-product (1%) in the metabolism of acetylsalicylic acid , which is excreted by the kidneys .
presentation
Historically, gentisic acid was made from 5-bromosalicylic acid by melting it with sodium hydroxide .
Furthermore, in 1881 Ferdinand Tiemann succeeded in producing it by melting potassium hydroxide with gentisinaldehyde , which was produced from hydroquinone and chloroform in the presence of sodium hydroxide solution.
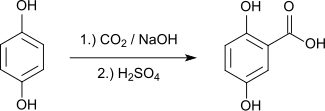
Gentisic acid is produced by the carboxylation of hydroquinone using the Kolbe-Schmitt reaction .
The synthesis is also possible at atmospheric pressure by boiling hydroquinone with carbon tetrachloride and copper powder . Tetrachloromethane is redox equivalent to carbon dioxide.
The oxidation of salicylic acid with potassium persulfate also produces gentisic acid in the presence of iron (II) sulfate .
Reactions
Direct bromination of gentisic acid yields 3-bromogenetic acid. An excess of bromine leads to the elimination of the carboxy group , resulting in bromanil (2,3,5,6-tetrabromo-1,4-benzoquinone). When the bromogenetic acid is heated to 160 ° C in the presence of water, bromohydroquinone is formed .
Acetylation with acetic anhydride yields diacetylgentisic acid, which melts at 118–119 ° C. With diazomethane can Methylester be established. The direct nitration of gentisic acid is only possible with low yields with very dilute nitric acid ; mainly an oxidation with the formation of oxalic acid takes place. The direct nitration of the diacetyl derivative also fails. The synthesis of 3-nitrogentisic acid can, however, take place via the methyl ester of diacetylgentisic acid. Reduction with tin (II) chloride and hydrochloric acid turns 3-nitrogentisic acid into 3-aminogentisic acid.
Like most hydroquinone derivatives, gentisic acid can be easily oxidized.
use
Gentisic acid is used as an intermediate in the manufacture of pharmaceuticals (especially analgesics and anti-inflammatory drugs ) and other organic compounds.
It is used in MALDI-TOF analysis as a matrix for ionizing peptides , proteins and carbohydrates . It can be used as a reagent for the detection of boric acid in peptides.
It is also used in medicine as glucosamide (amide of glucosamine ) for parenteral use. Gentisic acid, like salicylic acid , inhibits prostaglandin synthesis and was therefore previously used as an anti-inflammatory drug .
Sodium agent is used as a drug against rheumatic fever . Zinc and manganese gentisate can be used as catalysts for polyester formation . 2-aminogentisic acid has antibiotic properties against gram-positive and gram-negative bacteria.
Web links
2,5-dihydroxybenzoic acid
Individual evidence
- ↑ Entry on 2,5-DIHYDROXYBENZOIC ACID in the CosIng database of the EU Commission, accessed on May 13, 2020.
- ↑ Gentisic acid data sheet (PDF) from Merck , accessed on January 18, 2011.
- ↑ a b c d e Entry on gentisic acid in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Mary Eagleson: Concise Encyclopedia Chemistry . Walter de Gruyter, 1994, ISBN 3-11-011451-8 , p. 449 ( limited preview in Google Book search).
- ↑ a b Data sheet 2,5-Dihydroxybenzoic acid from Sigma-Aldrich , accessed on May 14, 2017 ( PDF ).
-
↑ a b entry on gentisic acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014
. - ↑ G. Levy, T. Tsuchiya: Salicylate Accumulation Kinetics in Man . In: New England Journal of Medicine . 287, No. 9, 1972, pp. 430-432, doi: 10.1056 / NEJM197208312870903 .
- ↑ P. v. Rakowski, W. Leppert: About Hydroquinonecarboxylic Acid . In: Reports of the German Chemical Society . 8, No. 1, 1875, pp. 788-790, doi: 10.1002 / cber.187500801256 , full text .
- ↑ F. Tiemann, M. Müller: Ueber Derivate des Hydrochinons . In: Reports of the German Chemical Society . 1881, 14 , pp. 1985-1999 full text .
- ↑ PM Hudnall: Hydroquinone . In Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2002, doi : 10.1002 / 14356007.a13_499 .
- ↑ J. Zeltner, Max Landau "Method for the preparation of phenol carboxylic acids", DE 258887 , filed January 10, 1912.
- ↑ MB Fawzi: Gentisic acid salts as radiographic scanning agent stabilizers . U.S. Patent 4,497,744 , filed November 3, 1980.
- ^ SG Morris: Preparation of Gentisic Acid and its Fatty Alcohol Esters in J. Am. Chem. Soc. 71, No. 6, 1949, pp. 2056-2057, doi: 10.1021 / ja01174a046 .
- ^ RL Crawford, SW Hutton, PJ Chapman: Purification and Properties of Gentisate 1,2-Dioxygenase from Moraxella osloensis . In: Journal of Bacteriology . 121, No. 3, 1975, pp. 794-799; PMC 246005 (free full text, PDF).
- ↑ MC Kloetzel, BY Abadir: Synthetic Analogs of Cortical Hormones. II. 3-Substituted α-2,5-Trihydroxyacetophenone Derivatives . In: J. Am. Chem. Soc. 77, No. 14, 1955, pp. 3823-3826, doi: 10.1021 / ja01619a043 .
- ↑ a b c d e F. v. Hemmelmayr: On the knowledge of gentisic acid (2, 5-dioxybenzenecarboxylic acid (1)) and some of its derivatives . In: Monthly magazine for chemistry . 30, No. 3, 1909, pp. 255-269, doi: 10.1007 / BF01519683 .
- ↑ a b A. Klemenc: About nitrogentisinic acids . In: Monthly magazine for chemistry . 1912, 33 , pp. 1243-1254, doi: 10.1007 / BF01518823 .
- ↑ F. v. Hemmelmayr: About some new derivatives of di- and trioxybenzoic acids . In: Monthly magazine for chemistry . 1914, 35 , pp. 1-8, doi: 10.1007 / BF01519727 .
- ↑ K. Strupat, M. Karas, F. Hillenkamp: 2,5-Dihydroxybenzoic acid: a new matrix for laser desorption — ionization mass spectrometry . In: International Journal of Mass Spectrometry and Ion Processes . 1991, 111 , pp. 89-102, doi : 10.1016 / 0168-1176 (91) 85050-V .
- ↑ JB Crumpton, W. Zhang, WL Santos: Facile Analysis and Sequencing of Linear and Branched Peptide Boronic Acids by MALDI Mass Spectrometry . In: Analytical Chemistry . 83, No. 9, 2011, pp. 3548-3554, doi: 10.1021 / ac2002565 .
- ↑ BW Meade AND MJH Smith: The estimation of Sodium gentisates in plasma and urine . In: Journal of Clinical Pathology . 1951, 4 , p. 226; PMC 1023403 (free full text, PDF).
- ↑ LE Schaefer, IA Rashkoff, RS Megibow: Sodium Gentisate in the Treatment of Acute Rheumatic Fever (PDF file; 1.18 MB). In: Circulation . 1950, 2 , pp. 265-270.
- ↑ JA Price: Zinc and manganese gentisate as polyester catalysts and molecular weight enhancer , US Patent No. 3673157 n filed July 31, 1970.
- ↑ A. Zeeck, S. Breiding-Mack, S. Grabley, H. Voelskow, G. Seibert (Hoechst AG): Gentisic acid derivatives having antibiotic activity . U.S. Patent 4,861,796 , filed December 19, 1988.