Glatiramer acetate
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|||||||||
| General | |||||||||
| Surname | Glatiramer | ||||||||
| other names |
|
||||||||
| CAS number |
|
||||||||
| Monomers / partial structures | L - alanine , L - glutamic acid , L - lysine , L - tyrosine | ||||||||
| Molar mass estimation |
5,000 to 9,000 daltons |
||||||||
| PubChem | 3081884 | ||||||||
| Type of polymer | |||||||||
| ATC code | |||||||||
| DrugBank | DB05259 | ||||||||
| Drug information | |||||||||
| Drug class | |||||||||
| properties | |||||||||
| safety instructions | |||||||||
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
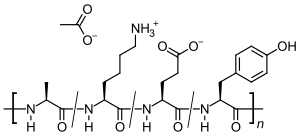
Glatiramer acetate ( GA ) is the acetic acid salt ( acetate ) of a synthetically produced polypeptide that contains the four naturally occurring amino acids L - alanine , L - glutamic acid , L - lysine and L - tyrosine in random order. The immunomodulatory active substance is used as a medicinal substance for the treatment of relapsing multiple sclerosis (MS). The exact mechanism of action is unknown. Since the composition of Glatiramer is similar to the components used to isolate nerve cells , it is said to reduce the inflammatory reactions in the central nervous system that occur in MS .
A glatiramer acetate injection solution was first marketed as Copaxone in 1996 in the USA by Teva . It was approved in the EU in 2001.
Clinical information
Approved areas of application
Glatiramer acetate reduces the number of relapses in ambulatory patients with relapsing multiple sclerosis . However, it does not prevent disability progression in patients with MS. It has also not been shown that treatment with glatiramer acetate reduces the duration or severity of an attack.
In the EU, glatiramer acetate is approved for the treatment of relapsing multiple sclerosis.
- Reduction of the frequency of relapses in unaided patients with relapsing-remitting multiple sclerosis (MS). In clinical trials, this was characterized by at least two relapses of neurological dysfunction in the past two years.
- Treatment of patients with a well-defined first clinical episode who are at high risk of developing clinically established multiple sclerosis. This additional indication - treatment of MS that has not yet been confirmed - was approved in 2009.
There is no approval for the primary or secondary progressive forms of MS.
The limited published data suggest that the safety profile in adolescents 12 to 18 years of age receiving glatiramer acetate 20 mg subcutaneously daily is comparable to that in adults.
Type and duration of application
Glatiramer acetate is injected daily or three times a week, depending on the active substance content of the pre-filled syringes, subcutaneously , i.e. into the subcutis . Treatment of MS requires long-term use.
unwanted effects
Local skin reactions such as reddening, burning, itching or hardening of the corresponding area of the subcutis are most common. These can be alleviated by cooling the injection site before and after the injection.
A side effect known as SPIR (Immediate Post Injection Reaction) or “flush” can rarely occur for an unexplained cause. Immediately after the injection, for a short time (approx. 5 to 15 minutes) there is shortness of breath, anxiety, feelings of fear, sweating, racing heart and then chills and headaches. The symptoms gradually subside over a period of twelve hours.
Studies
Beginnings
In 1977 the Weizmann Institute carried out the first open studies with the active ingredient in humans. Initially on four patients with advanced multiple sclerosis, followed by four patients with relapsing-remitting MS and twelve with chronic progressive disease. The results were encouraging enough to embark on a randomized controlled trial .
The planning of this pilot study was done by Murray B. Bornstein (1917-1995), from 1966 until his retirement in 1988 faculty member of the Albert Einstein College of Medicine in New York City . The primary endpoint of the study was to determine the extent to which patients would remain relapse-free. Secondary endpoints were the frequency of exacerbations and the degree of disability at the end of the study. 50 patients with relapsing-remitting MS were randomized and the study was completed with 48 participants. Since too little information about the stability of the Cop-1 was available, the patients received the preparation in a frozen state, allowed the daily ration to thaw and injected it themselves (after receiving appropriate training) once a day for two years. Under these conditions, 23 patients received placebo (saline solution) and 25 patients received Cop-1. The results were published in 1987 in the NEJM . In the placebo group there were 62 exacerbations, in the verum group there were 16. While the degree of disability in the placebo group worsened by 1.2 units, the degree of disability in the verum group improved by 0.5 units.
In 1996 the United States Adopted Name Council in Chicago introduced the name Glatiramer , whereby the first letters of the four amino acids involved and the ending "-mer" (standing for polymer) were used to name the international non-proprietary name in order to comply with the organization's guidelines for to match simple and understandable terms.
Clinical efficacy in relapsing-remitting MS
In the approval study with 251 patients over a treatment period of two years, a reduction in the relapse rate of around 30% compared to placebo was demonstrated.
A long-term study that emerged from the registration study has shown that the relapse rate continues to decrease over a longer observation period. The currently current 15-year data of the openly conducted prospective study continue this development. The relapse rate was reduced from around 1.2 relapses per year before the start of therapy by more than 80 percent to approximately 0.2 relapses per year in those patients who had received GA for more than 15 years. At the same time, the therapy had a positive effect on the neurological handicap: 57 percent of the patients who had been continuously treated with GA had a stable or improved value on the EDS scale after 15 years. This scale provides information about the degree of disability an MS patient has. More than 80 percent remained below an EDS value of 6, meaning that they were able to walk at least 100 meters independently without a walking aid. In those patients who had stopped treatment prematurely and were examined 10 years after the start of therapy, the degree of disability had remained stable or had improved in only 28 percent of the cases. It should be noted that the number of participants in the follow-up period of originally 232 people who had received glatiramer acetate at least once, only 100 patients continued the treatment after fifteen years. Of the 124 patients who dropped out, 50 returned to the treating clinic for the follow-up examination of the study after 10 years. It can be assumed that it was mainly those people who left the study in whom GA worked less well, at least part of the significantly lower relapse rate should be related to this. On the other hand, people can also refuse to participate in the study if their symptom-free status enables them to suppress their diagnosis.
The results of a meta-analysis from three placebo-controlled double-blind studies confirm the positive effect of GA on the relapse rate. In the course of the clinical investigations, a reduction in the annual relapse rate of 28 percent was found in those of the total of 540 study participants who were treated with the preparation, compared to those who only received a dummy drug. Treatment with GA also increased the time to the first attack (322 days versus 219 days with placebo). The risk of the degree of disability increasing significantly was also lower under GA, although the informative value of the analysis is limited on this point due to the relatively short duration of the studies (24, 35 and 9 months).
In an open study, the therapeutic success of GA and standard beta interferons in patients with relapsing MS was compared retrospectively. For this purpose, data from 308 patients were evaluated, which came from a database in which the two-year course of treatment is documented: After six months of treatment, the interferons and GA were comparable in their effectiveness in terms of reducing the number of attacks. After 12 and 24 months there were no differences between the interferon preparations. However, treatment with GA enabled the number of attacks to be reduced significantly more than with any of the interferons examined during this period. In addition, the dropout rate was lower among GA. It should be noted about this study that the informative value of the results is limited due to the nature of the study design.
In three other current studies - REGARD, BEYOND and BECOME - different beta-interferon preparations and GA were compared with one another. In the course of the randomized studies, in which a total of more than 3000 patients with relapsing MS were involved, a similarly good effectiveness was found for interferons and GA with regard to the time to the first attack, the relapse rates and the number of active lesions in the CNS.
Clinical efficacy in primarily progressive MS
A recent, controlled clinical study investigated the effectiveness of GA on primary progressive MS, in which there are no relapses but rather the symptoms progress chronically from the beginning. In the course of the study, there was no significant effectiveness of the therapy on the progression rate of the entire study population. However, the overall progression rate was lower than expected, which impaired the validity of the study. In contrast, the results of a retrospective analysis showed a clear treatment effect on the male subpopulation of the study, in which the progression rate was at the originally expected level.
Neuroprotective effect
The neuroprotective effect of GA was also shown in an MRI-based study. The number of lesions that change from scarred inflammation centers to irreversible axonal damage - so-called "black holes" - could be halved with the administration of GA. However, the protective effect of GA only takes effect after a treatment period of about six months.
The results of a study by Omar Khan also show that GA inhibits the loss of nerve tissue. He treated 18 relapsing MS patients with GA for six years from the onset of the disease. Other patients initially remained untreated. Regular measurements of the neural marker N-acetylaspartate (NAA) using magnetic resonance spectroscopy (MRS) showed that the ratio of NAA and creatine increased significantly in the treated patients . This in turn suggests a recovery in axonal function and protection from axonal damage. In the untreated patients, on the other hand, the NAA / creatine ratio decreased significantly, that is, a considerable loss of axons was found in these patients. This improved after starting therapy. Many patients develop antibodies to glatiramer acetate; however, their clinical significance is unknown.
Mechanism of action
How glatiramer acetate is able to favorably influence the course of MS has not been clarified with certainty; the following possible mechanisms of action are currently being discussed:
- The chemical structure of GA is very similar to that of myelin basic protein (MBP) - especially of sequence section 82 to 100 - a main component of the myelin layer . Cellular fragmentation into individual epitopes results in GA peptides that also have a high affinity for the MHC class I and MHC class II complex on dendritic cells . In contrast to MBP, however, the T cell receptor, which is complementary to the MHC, is not an agonist , but an antagonist .
- In addition, GA causes a shift in the ratio of T H 1 to T H 2 immune cells ( T helper cells ). The activated T H 2 cells cross the blood-brain barrier and increasingly release anti-inflammatory cytokines ( interleukin-4 , IL-6 and interleukin-10 ) in the CNS . At the same time, the production of pro-inflammatory cytokines such as IL-12 is reduced. This leads to the suppression of the pathological inflammatory processes in the MS lesion on the spot. In addition, the formation of neurotrophic factors, e.g. B. the Brain Derived Neurotrophic Factor (BDNF), promoted with a possible neuroprotective effect - axon loss can be inhibited and the destroyed myelin layer can be rebuilt.
- GA promotes the development of regulatory CD8 immune cells, which are usually significantly reduced in untreated MS patients.
- By activating the transcription factor FOXP3 ( forkhead box protein 3 ), GA promotes the conversion of conventional CD4 + CD25 T cells into CD4 + CD25 + regulatory T cells .
properties
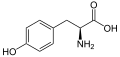
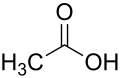
Glatiramer is a heterogeneous mixture of synthetic polypeptides . These consist of four natural amino acids glutamic acid , lysine , alanine and tyrosine ("GLAT"), which are present in a fixed molar ratio of 0.14: 0.34: 0.43: 0.09. The mean molar mass of the acetic acid salt glatiramer acetate (GA) is 5,000 to 9,000 Daltons .
Structural formulas of the amino acids contained in the peptide and the salt former acetic acid (acetate):
Since glatiramer acetate is randomly polymerized from the four amino acids it contains, which are only present in a certain ratio, the effectiveness of every batch that is sold must first be checked in animal experiments.
The substance is one of the non-biological complex drugs .
Other Information
history
In the 1960s, Michael Sela and Ruth Arnon carried out several series of experiments at the Weizmann Institute in Israel, which were intended to clarify the minimum chemical requirements a molecule must have in order to trigger an immune reaction in the organism, i.e. lead to the formation of antibodies . In addition, statements should be made about how the specificity of the immune reactions comes about . For this purpose, gelatine was equipped with short peptides of different compositions, whereby the immunogenicity of the gelatine could be significantly increased if tyrosine, tryptophan , phenylalanine or - here to a lesser extent - cysteine was added, while a covalent bond of peptides, alanine, lysine, glutamic acid , Serine and proline was not the case Later they realized that the peptide components and not the gelatine were responsible for the immunogenic properties, and that such synthetically produced molecules from 4000 Daltons are potent antigens . Subsequently, a polypeptide was also synthesized that was supposed to be similar to myelin basic protein (MBP ) in order to trigger experimental autoimmune encephalomyelitis (EAE) in test animals , i.e. an auto-immune reaction against MBP.
Three polypeptides were produced which were formed by copolymerization and were therefore referred to as copolymers.
While none of the polypeptides produced could provoke EAE, it was instead observed and published in 1971 that EAE was suppressed in the domestic guinea pigs used in this series of experiments . In this case, all three copolymers showed this protective effect when the aid of Freund's adjuvant , a strong immune-stimulating and irritating adjuvant , intradermally administered. Only Copolymer-1 showed an effect after intravenous injection with saline solution . This simplest of the three copolymers also had the fewest side effects. Copolymer-1 or Cop-1 for short , which kept this working name until 1996, should therefore be decisive for further development.
As a result, it was possible to demonstrate that this protective effect was not limited to individual animal families and existed independently of the type of trigger used for EAE.
As Stanley Scheindlin states, the researchers were now convinced that instead of a pharmacological agent they had found a potential drug. Further preclinical studies on the safety of the substance followed, with laboratory animals ( mice , rats , rabbits and beagles ) being administered different doses for different periods of time.
The commercial exploitation of the research successes and approval under the name Copaxone took place in 1996 in the United States and in November 2001 for all member states of the European Union by the companies TEVA and Sanofi-Aventis , so that it was available in Austria from January 2002, for example. The name Copaxone also makes several references to the history of the substance's development: 'Cop' for copolymer, 'ax (on)' for nervous (ensystem) and 'one' for (copolymer) 1.
Trade names and dosage forms
- Copaxone ( Teva Pharma )
- Glatopa ( Sandoz Inc., USA)
- Glatiramyl (BGP Products GmbH, CH )
Solution in pre-filled syringes for subcutaneous injection
costs
The annual costs in 2011 amounted to EUR 11,600 (source: cost list of the Upper Austrian regional health insurance fund OÖGKK)
Web links
- Detailed scientific discussion of the mechanism of action of GLT (Engl.)
- Stanley Scheindlin: Copolymer 1: An Off-Beat Drug Development Story ( PDF ). About the development history of Copaxone. In: Molecular Interventions, 2014
- Barbara Peruche, Martin Schulz: Glatiramer acetate for the treatment of multiple sclerosis , Pharmazeutische Zeitung, issue 34/2002.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Copaxone technical information, as of February 2009.
- ↑ a b c d e S. Scheindlin: Copolymer 1: an off-beat drug development story. In: Molecular interventions. Volume 4, number 1, February 2004, pp. 6-9, doi : 10.1124 / mi.4.1.6 . PMID 14993470 .
- ↑ MB Bornstein, A. Miller u. a .: A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. In: The New England Journal of Medicine . Volume 317, Number 7, August 1987, pp. 408-414, doi : 10.1056 / NEJM198708133170703 . PMID 3302705 .
- ↑ CC Ford u. a .: A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis. In: Multiple Sclerosis. 12, 2006, pp. 309-320.
- ↑ CC Ford u. a .: Continuous Long-Term Immunomodulatory Therapy in Relapsing Multiple Sclerosis: Results from the 15-Year Analysis of the US Prospective Open-label Study of Glatiramer Acetate. In: Multiple Sclerosis. 14, 2008, p. 41.
- ↑ FM Boneschi u. a .: Effects of glatiramer acetate on relapse rate and accumulated disability in multiple sclerosis: meta-analysis of three double-blind, randomized, placebo-controlled clinical trials. In: Multiple Sclerosis 9, 2003, pp. 349-355.
- ↑ J. Haas et al. a .: Twenty-four-month comparison of immunomodulatory treatments - a retrospective open label study in 308 RRMS patients treated with beta interferons or glatiramer acetate (Copaxone) . In: European Journal of Neurology. 12, 2005, pp. 425-431.
- ↑ JS Wolinsky et al. a .: Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. In: Ann Neurol. 61, 2007, pp. 14-24.
- ↑ M. Filippi et al. a .: Glatiramer acetate reduces the proportion of new MS lesions evolving into "black holes". In: Neurology. 57, 2001, pp. 731-733.
- ↑ O. Khan et al. a .: Axonal metabolic recovery and potential neuroprotective effect of glatiramer acetate in relapsing-remitting multiple sclerosis. In: Mult Scler. 11, 2005, pp. 646-651.
- ↑ O. Khan et al. a .: Long-term evaluation of neuronal metabolism in relapsing-remitting multiple sclerosis: combined results from two cohorts of glatiramer acetate treated patients examined prospectively with serial brain proton magnetic resonance spectroscopy (1H-MRS) . In: Abstract presented at the 18th Meeting of the European Neurological Society ENS. June 7th to 11th, 2008, in Nice
- ↑ Cohen BA, Oger J, Gagnon A, Giovannoni G. The implications of immunogenicity for protein-based multiple sclerosis therapies. J Neurol Sci. 2008; 275, 7-17, PMID 18822434
- ↑ R. Aharoni, D. Teitelbaum u. a .: Copolymer 1 acts against the immunodominant epitope 82-100 of myelin basic protein by T cell receptor antagonism in addition to major histocompatibility complex blocking. In: PNAS . Volume 96, Number 2, January 1999, pp. 634-639, PMID 9892685 . PMC 15188 (free full text).
- ↑ M. Ruggieri, C. Avolio et al. a .: Glatiramer acetate in multiple sclerosis: a review. In: CNS Drug Reviews . Volume 13, Number 2, 2007, pp. 178-191, doi : 10.1111 / j.1527-3458.2007.00010.x . PMID 17627671 . (Review).
- ↑ C. Farina, MS Weber et al. a .: Glatiramer acetate in multiple sclerosis: update on potential mechanisms of action. In: The Lancet Neurology . Volume 4, Number 9, September 2005, pp. 567-575, doi : 10.1016 / S1474-4422 (05) 70167-8 , PMID 16109363 .
- ↑ J. Hong, N. Li et al. a .: Induction of CD4 + CD25 + regulatory T cells by copolymer-I through activation of transcription factor Foxp3. In: Proceedings of the National Academy of Sciences of the United States of America . Volume 102, Number 18, May 2005, pp. 6449-6454, doi : 10.1073 / pnas.0502187102 . PMID 15851684 , PMC 1088385 (free full text).
- ↑ Prescribing Information , copaxone.com (PDF, English).
- ↑ Glatiramer acetate ( Copaxone dry matter; Aventis) ; Pharmaceutical newspaper , issue 51/2001.
- ↑ M. Sela, S. Fuchs, R. Arnon: Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine. In: The Biochemical journal. Volume 85, October 1962, pp. 223-235, PMID 13992690 . PMC 1243934 (free full text).
- ↑ D. Teitelbaum, A. Meshorer u. a .: Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. In: European Journal of Immunology. Volume 1, Number 4, August 1971, pp. 242-248, doi : 10.1002 / eji.1830010406 . PMID 5157960 .
- ↑ D. Teitelbaum, C. Webb u. a .: Suppression by several synthetic polypeptides of experimental allergic encephalomyelitis induced in guinea pigs and rabbits with bovine and human basic encephalitogen. In: European Journal of Immunology. Volume 3, Number 5, May 1973, pp. 273-279, doi : 10.1002 / eji.1830030505 . PMID 4128127 .
- ↑ D. Teitelbaum, C. Webb u. a .: Suppression of experimental allergic encephalomyelitis in Rhesus monkeys by a synthetic basic copolymer. In: Clinical immunology and immunopathology. Volume 3, Number 2, November 1974, pp. 256-262, PMID 4141659 .
- ↑ ANDA: 090218