Human rotaviruses
| Rotaviruses | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
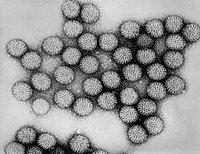
Human rotavirus |
||||||||||||||||||||
| Systematics | ||||||||||||||||||||
|
||||||||||||||||||||
| Taxonomic characteristics | ||||||||||||||||||||
|
||||||||||||||||||||
| Scientific name | ||||||||||||||||||||
| Rotavirus | ||||||||||||||||||||
| Short name | ||||||||||||||||||||
| RVA to RVI | ||||||||||||||||||||
| Left | ||||||||||||||||||||
|
The human rotaviruses are viruses of the genus Rotavirus (family Reoviridae , subfamily Sedoreovirinae ) occurring in humans . The name is based on the wheel-like structure (lat. Rota = the wheel) of viruses under the electron microscope . They were discovered in 1973 by Ruth Bishop in small intestine biopsies from sick children. Rotaviruses are the most common cause of severe diarrhea , contributing to around 140 million cases worldwide. Rotaviruses account for 35–52% of clinically relevant diarrheal diseases. The World Health Organization estimates that 527,000 children under the age of 5 die each year from rotavirus-induced dehydration .
Rotaviruses are also widespread in the animal kingdom . In the veterinary field, rotavirus infections of calves are of great economic importance.
Structure of the rotaviruses
Human rotaviruses are 76 nm RNA viruses with a double-shell icosahedral capsid . There is no virus envelope . The genome consists of eleven double-stranded RNA segments from 0.6 to 3.3 kb in length. Each of these segments encodes a viral protein. The segmentation of the genome makes it possible to generate reassortants .
classification
Human rotaviruses belong to the Reoviridae family , subfamily Sedoreovirinae . A total of nine species are known within the genus Rotavirus , designated Rotavirus A to Rotavirus I ( International Committee on Taxonomy of Viruses (ICTV), as of 2020). The species classification is complex and includes serotypes and subtypes, so species A to C contain at least eleven serotypes and subtypes which are pathogenic to humans.
Humans are primarily infected with species A, B and C, most frequently with species A. The latter is therefore the most clinically important. Species A through E also cause disease in animals, such as species E and H in pigs, species D, F and G in birds, and species I in cats.
For the serotypes within the species Rotavirus A , a dual classification system similar to that of the influenza virus Influenzavirus A is used, which is based on two proteins on the surface of the virion . The glycoprotein VP7 defines the G serotypes and the protease- sensitive protein VP4 defines the P serotypes.
Since the two genes that determine G and P types can be passed on to the daughter viruses separately , different combinations are found - as is the case with influenza . For group A rotaviruses, a typing system was set up for the entire genome , which can be used to determine the origin of atypical lines. The distribution of rotaviruses of the individual G and P types also varies from country to country and year to year, as does the H / N types of flu viruses. The G and P antigen types can be combined variably.
transmission
The infection with rotaviruses usually takes place in the traditional faecal - oral manner , whereby contaminated food and contaminated drinking water can play a role. Transmission by aerosols via the air is possible experimentally. After a serious illness, the pathogen is usually excreted for about one to three weeks. However, elimination is possible up to eight weeks after illness. Longer elimination is possible in patients with immunodeficiency. The pathogen can survive for days on surfaces and for weeks in water (high tenacity ). The pathogen can also survive on hands.
A high level of infection already in childhood is due to the low infection dose of only 10 virus particles, the high concentration density in diarrhea (around 10 11 infectious particles per milliliter) and the high environmental tolerance of the pathogen.
Epidemiology
Rotaviruses are spread around the world. By the end of the third year of life, most children (> 90%) have already had a rotavirus infection. In Germany, an average of 50,000 cases of illness are reported each year. Most cases of the disease occur between February and April ( temperate zones ). In contrast, diseases occur year-round in the tropics . In the course of the first few years of life, antibodies are increasingly formed as a result of contact with rotaviruses. Earlier illnesses can protect against recurrence in the event of a later reinfection with the same or different rotavirus types. It is true that you get sick only slightly, if at all, but infected people excrete infectious viruses and are thus carriers. In adulthood, diseases occur primarily as traveler's diarrhea , with only about 20% of traveler's diarrhea being caused by rotaviruses. The most severe course of the disease can be found in the age group between six months and two years. In the temperate climates, rotavirus infections are mainly seen during the winter months. With the exception of children, serious illnesses from rotavirus infection have occurred in the elderly or in the immunosuppressed.
Diseases and Physiology
The clinical symptoms appear after an incubation period of one to three days. However, the infection can also be clinically inapparent, i.e. H. occur without symptoms. Symptoms often begin with vomiting , followed by moderate fever and non-bloody diarrhea , but abdominal pain is rare. In severe disease, the diarrhea can last four to five days and lead to desiccosis due to the resulting loss of water and electrolytes , which can be potentially life-threatening. This and the metabolic acidosis that occurs can lead to cardiac arrhythmias and even cardiac arrest . The usual duration of illness is six to eight days. In small children, infection with rotaviruses can, as a particular complication, lead to intussusception of the intestine, which must be treated surgically. The viruses can also get into the blood ( viraemia ) and attack the kidneys and liver .
The pathogen multiplies in the apical enterocytes of the small intestinal villi. The effect is presumably increased by the viral protein (NSP4), which has the properties of an enterotoxin.
After about 10 to 14 days, virus excretion stops , whereas immunocompromised people suffer from diarrhea and related virus excretion for months.
diagnosis
The diagnostic examination for rotaviruses is usually carried out from the stool with an immunassay that specifically detects a capsid protein as an antigen . Antigen tests often have only low analytical sensitivity . However, in the acute phase of the disease, there is a lot of viral antigen in the liquid stool, so that the test sensitivity is sufficient to confirm rotaviruses as the cause. The virus cannot be confirmed by means of an antigen test if it is excreted over a long period without acute signs of infection due to the low amount of antigen.
Detection in the stool using electron microscopy is easy , since the rotaviruses can be easily recognized by their typical morphology. RT-PCR is recommended for specific questions about the elimination status or to determine the subtypes for epidemiological investigations . Classical virus isolation , RNA electrophoresis or nucleic acid hybridization reactions are only rarely used.
Rapid test methods for the detection of antigens are possible, but their use is limited due to their low sensitivity and insufficient specificity (false positive results). Serological methods for the detection of specific antibodies against rotaviruses are of no diagnostic significance.
prophylaxis
As a prophylactic measure, compliance with general hygiene standards and the isolation of patients when admitted to hospital, possibly in cohort isolation, are used . Due to the high tenacity of the pathogen is a high compliance essential for surface disinfection should virucidal agents.
vaccination
There are two different rotavirus vaccines available in Europe : A monovalent vaccine (Rotarix® from GlaxoSmithKline ) - RV1 - and a pentavalent vaccine RV5 ( RotaTeq from Sanofi Pasteur MSD ). The vaccination schedule consists of two or three partial vaccinations. These are oral vaccinations . The immunization must be completed at the end of the 24th ( Rotarix ) or 32nd ( RotaTeq ) week of life. In August 2013, the rotavirus vaccination was added to the STIKO vaccination calendar and is recommended in Germany for babies from the age of six weeks. Without a vaccination, almost every child will develop rotaviruses by the age of five. In addition to protection from rotavirus infection, there is also evidence in the United States of a decrease in seizures after rotavirus vaccination.
A third, monovalent vaccine has been approved in India since 2014: Rotavac. RV1, RV5 and Rotavac are pre-qualified by the WHO and are considered safe and effective.
Oral vaccination against rotaviruses ( RotaShield by Wyeth Lederle ) was included in the normal vaccination schedule in the USA in 1998, but withdrawn on October 15, 1999 after 76 cases of intussusception ( bowel invagination ) nationwide and a possible connection with the vaccination was indicated . After intensive clinical studies, rotavirus vaccinations for children up to six months of age have been approved again in Europe and the USA since the second quarter of 2006. A clinical study on Rotarix - with 615 examined intussusceptions from Brazil and Mexico - was published in the New England Journal of Medicine in June 2011 . In the case control study , a slightly increased risk was found, but a positive risk-benefit balance for both countries.
According to a study by the Australian Therapeutic Goods Administration (without differentiating the time of vaccination) the risk of intussusception increased by a factor of 3.5 (0.7-10.1), while for RotaTeq a risk increased by a factor of 5.3 ( 1.1-15.4) was found.
Harry Greenberg from Stanford University interpreted the results in the NEJM to the effect that "Intussusceptions are a principal risk of all rotavirus infections", since both vaccines consist of live viruses. Because vaccination leads to inapparent infections, it could be that the risk of disease from vaccination is lower than with infection with the wild type. The risk is considered to be very low, however, for every 100,000 vaccinated infants there are likely to be 1 to 5 incidents.
therapy
There is no special therapy. In any case, ensure that there is sufficient fluid intake (electrolyte solution, if necessary). It is not advisable to take antidiarrheal drugs because they make it difficult to eliminate the pathogen and thus prolong the course of the disease.
Reporting requirement
In Germany, proof of rotaviruses must be reported by name in accordance with Section 7 of the Infection Protection Act (IfSG) . In addition, according to § 6 IfSG, suspected and [...] illness of acute infectious gastroenteritis if the person concerned is handling food, works in a community facility or if an outbreak is to be suspected based on the epidemic context ( if two or more similar diseases occur for which an epidemic connection is likely or suspected ). In addition, in accordance with Section 34 (6) and (1) IfSG, the management of communal facilities must immediately notify the responsible health department if children cared for in their facility who have not yet reached the age of 6 and are sick with or are suspected of having infectious gastroenteritis .
Web links
- Rotavirus infections - information from the Robert Koch Institute
Individual evidence
- ↑ ICTV Master Species List 2018b v1 MSL # 34, Feb. 2019
- ↑ a b c d e ICTV: Bluetongue virus , EC 51, Berlin, Germany, July 2019; Email ratification March 2020 (MSL # 35)
- ↑ a b c d e f g h i j k l Cornelia Henke-Gendo: Viral gastroenteritis pathogens . In: Sebastian Suerbaum, Gerd-Dieter Burchard, Stefan HEKaufmann, Thomas F. Schulz (eds.): Medical microbiology and infectious diseases . Springer-Verlag, 2016, ISBN 978-3-662-48678-8 , pp. 513 ff ., doi : 10.1007 / 978-3-662-48678-8_65 .
- ↑ RF Bishop et al .: Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis . In: Lancet (London, England) . tape 2 , no. 7841 , December 8, 1973, p. 1281-1283 , doi : 10.1016 / s0140-6736 (73) 92867-5 , PMID 4127639 .
- ↑ WHO | Rotavirus. In: www.who.int. Retrieved December 9, 2018 .
- ↑ Ulrich Dessel Berger: rotaviruses . In: Virus Research . tape 190 , September 22, 2014, p. 75-96 , doi : 10.1016 / j.virusres.2014.06.016 .
- ↑ International Committee on Taxonomy of Viruses (ICTV). Accessed April 13, 2020 (English).
- ^ Carl D. Kirkwood: Genetic and antigenic diversity of human rotaviruses: potential impact on vaccination programs . In: The Journal of Infectious Diseases . 202 Suppl, September 1, 2010, p. S43-48 , doi : 10.1086 / 653548 , PMID 20684716 .
- ↑ Mitsutaka Wakuda et al .: Porcine rotavirus closely related to the novel group of human rotaviruses . In: Emerging Infectious Diseases . tape 17 , no. 8 , August 2011, p. 1491–1493 , doi : 10.3201 / eid1708.101466 , PMID 21801631 , PMC 3381553 (free full text).
- ↑ Douglas Marthaler et al .: Widespread rotavirus H in commercially raised pigs, United States . In: Emerging Infectious Diseases . tape 20 , no. 7 , July 2014, p. 1195–1198 , doi : 10.3201 / eid2007.140034 , PMID 24960190 , PMC 4073875 (free full text).
- ^ Tung G. Phan et al .: Rotavirus I in feces of a cat with diarrhea . In: Virus Genes . tape 53 , no. 3 , June 2017, p. 487-490 , doi : 10.1007 / s11262-017-1440-4 , PMID 28255929 , PMC 7089198 (free full text).
- ^ Miguel O'Ryan: The ever-changing landscape of rotavirus serotypes . In: The Pediatric Infectious Disease Journal . tape 28 , 3 Suppl, March 2009, pp. S60-62 , doi : 10.1097 / INF.0b013e3181967c29 , PMID 19252426 .
- ↑ there classified with H and N.
- ^ A b John T. Patton: Rotavirus diversity and evolution in the post-vaccine world . In: Discovery Medicine . tape 13 , no. 68 , January 2012, p. 85-97 , PMID 22284787 , PMC 3738915 (free full text).
- ^ My VT Phan et al .: Unbiased whole-genome deep sequencing of human and porcine stool samples reveals circulation of multiple groups of rotaviruses and a putative zoonotic infection . In: Virus Evolution . tape 2 , no. 2 , July 2016, p. vew027 , doi : 10.1093 / ve / vew027 , PMID 28748110 , PMC 5522372 (free full text).
- ↑ Beards GM, Desselberger U, Flewett TH: Temporal and geographical distributions of human rotavirus serotypes, 1983 to 1988 . In: Journal of Clinical Microbiology . Vol. 27, No. December 12 , 1989, pp. 2827-33 , PMID 2556435 , PMC 267135 (free full text).
- ^ DS Prince et al .: Aerosol transmission of experimental rotavirus infection . In: Pediatric Infectious Disease . tape 5 , no. 2 , March 1986, p. 218–222 , doi : 10.1097 / 00006454-198603000-00012 , PMID 3005999 .
- ↑ Simone Richardson, Keith Grimwood, Rebecca Gorrell, Enzo Palombo, Graeme Barnes: Extended excretion of rotavirus after severe diarrhea in young children . In: The Lancet . tape 351 , no. 9119 , June 20, 1998, pp. 1844-1848 , doi : 10.1016 / s0140-6736 (97) 11257-0 .
- ↑ Shou-Chien Chen, Lia-Beng Tan, Li-Min Huang, Kow-Tong Chen: Rotavirus infection and the current status of rotavirus vaccines . In: Journal of the Formosan Medical Association . tape 111 , no. 4 , April 1, 2012, p. 183–193 , doi : 10.1016 / j.jfma.2011.09.024 .
- ↑ SA Ansari, VS Springthorpe, SA Sattar: Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks . In: Reviews of Infectious Diseases . tape 13 , no. 3 , June 1, 1991, pp. 448-461 , PMID 1866549 .
- ^ Robert Koch Institute: SurvStat @ RKI 2.0. Retrieved May 31, 2016 .
- ↑ Rotavirus Vaccines. In: Paul Ehrlich Institute. March 23, 2020, accessed April 13, 2020 .
- ↑ New vaccination calendar: Experts recommend vaccinating against rotaviruses. In: Spiegel Online . August 26, 2013, accessed January 21, 2015 .
- ↑ Epidemiological Bulletin 35/2013 of the Robert Koch Institute
- ^ Matthias Thalhammer, Lisa Demel: Rotavirus - vaccination. In: Netdoktor.at . April 1, 2011, accessed December 9, 2018 .
- ↑ DC Payne, J. Baggs et al. a .: Protective association between rotavirus vaccination and childhood seizures in the year following vaccination in US children. In: Clinical infectious diseases. Volume 58, Number 2, January 2014, pp. 173-177, ISSN 1537-6591 . doi: 10.1093 / cid / cit671 . PMID 24265355 .
- ↑ a b Karla Soares-Weiser et al .: Vaccines for preventing rotavirus diarrhea: vaccines in use . In: The Cochrane Database of Systematic Reviews . tape 2019 , no. 10 , October 28, 2019, doi : 10.1002 / 14651858.CD008521.pub5 , PMID 31684685 , PMC 6816010 (free full text).
- ↑ MM Patel, VR López-Collada a. a .: Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. In: The New England Journal of Medicine . Volume 364, Number 24, June 2011, pp. 2283-2292, doi: 10.1056 / NEJMoa1012952 . PMID 21675888 .
- ↑ JP Buttery, MH Danchin et al. a .: Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. In: Vaccine. Volume 29, Number 16, April 2011, pp. 3061-3066, doi: 10.1016 / j.vaccine.2011.01.088 . PMID 21316503 .
- ↑ Rotavirus vaccination: studies confirm minimal risk of intussusceptions. In: Deutsches Ärzteblatt. February 10, 2014, accessed April 13, 2020 .
- ↑ RKI - RKI Guide for Doctors - Rotavirus Gastroenteritis. In: www.rki.de. May 1, 2010, accessed March 18, 2020 .