Cyhalothrin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
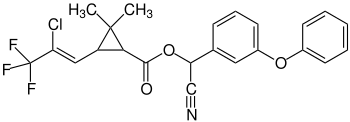
|
||||||||||
| Structural formula without specifying the stereochemistry | ||||||||||
| General | ||||||||||
| Non-proprietary name | Cyhalothrin | |||||||||
| other names |
3- (2-Chloro-3,3,3-trifluoro-1-propenyl) -2,2-dimethyl-cyclopropanecarboxylic acid cyano (3-phenoxyphenyl) methyl ester |
|||||||||
| Molecular formula | C 23 H 19 ClF 3 NO 3 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| ATC code | ||||||||||
| Drug class | ||||||||||
| Mechanism of action |
Opening of the Na + channels |
|||||||||
| properties | ||||||||||
| Molar mass | 449.86 g · mol -1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
1.33 g cm −3 |
|||||||||
| Melting point |
49.2 ° C |
|||||||||
| solubility |
practically insoluble in water (0.005 mg l −1 ) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data |
|
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Cyhalothrin is an insecticide from the group of pyrethroids used in veterinary medicine and in various agricultural and forestry crops . It is a white, odorless, crystalline substance that is hardly soluble in water. Cyhalothrin is a contact poison and also has an insect- repellent effect, which is long-lasting due to its low vapor pressure .
Stereochemistry
Cyhalothrin is a chiral compound that contains several asymmetrically substituted carbon atoms. The compound can therefore be present as a mixture of several isomers or enantiomerically pure .
The pure isomer ( S ) -α-cyano-3-phenoxybenzyl- ( Z ) - (1 R , 3 R ) -3- (2-chloro-3,3,3 ) is called gamma-cyhalothrin (γ-cyhalothrin) -trifluorprop-1-enyl) -2,2-dimethylcyclopropanecarboxylate:
Be with lambda-cyhalothrin (λ-cyhalothrin)
- ( S ) -α-Cyano-3-phenoxybenzyl- ( Z ) - (1 R , 3 R ) -3- (2-chloro-3,3,3-trifluoroprop-1-enyl) -2,2-dimethylcyclopropanecarboxylate and
- ( R ) -α-Cyano-3-phenoxybenzyl- ( Z ) - (1 S , 3 S ) -3- (2-chloro-3,3,3-trifluoroprop-1-enyl) -2,2-dimethylcyclopropanecarboxylate
designated.
properties
Cyhalothrin has a flash point of 81–85 ° C and a decomposition temperature of <250 ° C.
Mode of action
Insects ingest cyhalothrin through the body surface, whereupon it is distributed throughout the insect's body. It is a neurotoxin and causes the Na + channels of the nerve cells to no longer close. Na + ions flow unhindered into the inside of the cell and uncontrollable nerve impulses occur. This initially leads to states of excitement with cramps, then to coordination disorders and finally to paralysis. The insect is unable to move within a few minutes, which is known as a "knock-down" effect. The repellant effect is based on the irritation of tactile elements in the extremities (“foot retraction effect”) of the arthropods .
Death only occurs after a while. Ticks are killed within two days. Cyhalothrin's effects last for two weeks to five months.
If the dose is insufficient, many of the insects affected can break down cyhalothrin enzymatically (detoxification esterases and mixed function oxidase). By adding synergists such as piperonyl butoxide , enzymatic degradation can be prevented.
Areas of application
Veterinary medicine
Cyhalothrin is used to repel and combat stinging, biting and sucking-licking insects. It is administered either in the form of prepared ear tags (here the repellant effect predominates) or as an infusion ( pour-on ). Within a few hours it spreads to the animal's body via sweat and sebum. In ruminants is the active ingredient against ticks , horn flies ( Haematobia spp.), Head flies ( Musca autumnalis ), brakes , lice and lice , in pigs against lice and in poultry against bird lice and fleas effective.
In Regulation (EEC) No. 2377/90 on maximum levels for veterinary medicinal product residues in foodstuffs , the active substance is approved for cattle in Annex I. The maximum permissible residue level in kidneys and milk is 50 µg / kg, in fat 500 µg / kg. No veterinary preparation based on cyhalothrin is currently approved in Germany.
Plant protection
In crop protection, lambda cyhalothrin is used against insect infestation in agricultural and forestry crops as well as in vegetable growing. The agent is poisonous for fish and fish nutrients and has been classified as dangerous to bees (B1 / SPe 8). It is available as a capsule suspension or water- dispersible granulate .
Lambda-Cyhalothrin is approved in Switzerland, Austria and Germany against a variety of biting and sucking insects on grain, rapeseed, various fodder and oil plants, vegetables, tea herbs, hops, on soft fruit, in viticulture and in the forest. In organic agriculture , the insecticide may only be used as a lure in traps against the Mediterranean and olive fruit flies .
Gamma-cyhalothrin is approved in some EU countries, including Germany and Austria, as well as Switzerland.
The use of cyhalothrin is not permitted in the states of the EU or in Switzerland.
Trade names
- Veterinary medicine: Cyhalothrin ad us. vet. (CH)
- Plant protection: Karate Zeon , Karate Forst liquid ( Syngenta ), Trafo WG
Web links
- Entry on Cyhalothrin at Vetpharm
- WHO specifications and evaluations for public health pesticides: Lambda-Cyhalotrin , as of August 2015 (PDF; 360 kB; English)
Individual evidence
- ↑ a b c d e f g h Entry on lambda-cyhalothrin in the GESTIS substance database of the IFA , accessed on December 31, 2019(JavaScript required) .
- ↑ Entry to A mixture of: α-cyano-3-phenoxybenzyl (Z) - (1R, 3R) - [(S) -3- (2-chloro-3,3,3-trifluoro-prop-1-enyl) ] -2,2-dimethylcyclopropanecarboxylate; α-cyano-3-phenoxybenzyl (Z) - (1S, 3S) - [(R) -3- (2-chloro-3,3,3-trifluoro-prop-1-enyl)] -2,2-dimethylcyclopropanecarboxylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 31, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Stephen J. Maund, Michael J. Hamer, Jacqueline S. Warinton, Timothy J. Kedwards: Aquatic ecotoxicology of the pyrethroid insecticide lambda-cyhalothrin: considerations for higher-tier aquatic risk assessment. In: Pesticide Science . 54, 1998, pp. 408-417, doi : 10.1002 / (SICI) 1096-9063 (199812) 54: 4 <408 :: AID-PS843> 3.0.CO; 2-T .
- ↑ Entry on gamma-cyhalothrins in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on June 5, 2017.
- ↑ Entry on lambda-cyhalothrins in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on June 5, 2017.
- ↑ Data sheet for Axiendo pest-free spray, Federal Office for Consumer Protection and Food Safety
- ↑ FOAG - Karate Zeon - Products - Plant Protection Products Directory ( Memento from September 6, 2017 in the Internet Archive )
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on lambda-cyhalothrin in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on gamma-cyhalothrin in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 8, 2019.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on cyhalothrin in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.
- ↑ Jens Blankennagel: Controversial insecticide use in Brandenburg: From Monday, "Liquid Karate" will fall from the sky. In: berliner-kurier.de . May 3, 2019, accessed May 4, 2019 .
