Lorándite
| Lorándite | |
|---|---|
| Lorandite (red) and auripigment (yellow) from Allchar (Alsar), Roszdan, Macedonia (size: 3.4 cm × 2.7 cm × 2.4 cm) | |
| General and classification | |
| chemical formula | TlAsS 2 |
|
Mineral class (and possibly department) |
Sulfosalts |
|
System no. to Strunz and to Dana |
2.HD.05 ( 8th edition : II / E.13) 07/03/06/01 |
| Similar minerals | Cinnabarite , realgar , ruby |
| Crystallographic Data | |
| Crystal system | monoclinic |
| Crystal class ; symbol | monoclinic prismatic 2 / m |
| Room group (no.) | P 2 1 / a (No. 14) |
| Lattice parameters |
a = 12.27 Å ; b = 11.33 Å; c = 6.11 Å β = 104.2 ° |
| Formula units | Z = 8 |
| Physical Properties | |
| Mohs hardness | 2 to 2.5 |
| Density (g / cm 3 ) | 5.53 |
| Cleavage | excellent after {100}, very good after {201}, good after {001} |
| colour | scarlet red, tapering to lead gray |
| Line color | cherry red |
| transparency | translucent to transparent |
| shine | metallic |
| Crystal optics | |
| Refractive indices | n α = 2.720 |
| Birefringence | δ = 2.720 |
| Optical character | biaxial positive |
| Axis angle | 2V = strong |
| Pleochroism | weak: purple-red to orange-red |
Lorándite is a rarely occurring mineral from the mineral class of "sulfides and sulfosalts". It crystallizes in the monoclinic crystal system with the composition TlAsS 2 ( thallium , arsenic and sulfur ) and, according to the nomenclature of the International Mineralogical Association (IMA), is a binary sulfosalt with a cation / chalcogen ratio of 1: 1. According to the chemical nomenclature according to IUPAC Lorándite is a thallium (I) thioarsenite or a thallium (I) thioarsenate (III).
Lorándite usually develops granular to massive aggregates , in rare cases also well-formed, tabular or prismatic to needle-like crystals . Fresh samples are scarlet in color with a metallic sheen. Over time, however, the mineral often turns lead-gray. However, the color of the streak remains carmine red.
Lorándite is the most common thallium mineral .
Etymology and history
Lorándite was recognized and described as an independent mineral in 1894 by József Krenner, who named it after the Hungarian physicist Loránd Eötvös .
Synthetic crystals can be grown by hydrothermal transport fractions.
classification
In the meanwhile outdated, but still in use 8th edition of the mineral classification according to Strunz , the Lorándite belonged to the mineral class of "sulfides and sulfosalts" and there to the department of "sulfosalts", where together with Edenharterite , Grumiplucite , Hutchinsonite , Jentschit , Livingstonite , Simonite , Tvalchrelidzeit , Vaughanit , Vrbait and Weissbergit the unnamed group with the system no. II / E.13 formed.
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), also assigns Lorándite to the class of “sulfides and sulfosalts”, but there in the more finely subdivided section of “sulfosalts with SnS as Role model ”. This division is also further subdivided according to the predominant metals in the compound, so that the mineral can be found according to its composition in the sub-division “With Thallium (Tl)”, where it is the only member of the unnamed group 2.HD.05 .
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns Lorándite to the class of "sulfides and sulfosalts" and there in the department of "sulfosalts". Here he is the only member of the 03.07.06 group within the subdivision “ 03.07 Sulphosalts with the ratio z / y = 2 and the composition (A +) i (A2 +) j [ByCz], A = metals, B = semi-metals, C = Non-metals ”.
Education and Locations
Lorandite formed hydrothermally at low temperatures, and enters together with stibnite , realgar , Orpiment , cinnabar , Vrabit , Greigerit and marcasite , pyrite , tetrahedrite , sphalerite , solid arsenic and barite on. The type locality is Allchar in North Macedonia . Other known deposits are Hg-Tl-Au deposits in China ( Xiangquan Tl deposit, Lanmuchang Tl (Hg) -Lagerstätte, Zimudang Au-Hg (Tl) -Lagerstätte) and the USA ( Nevada , Utah , Wyoming ) Zarshuran gold deposit ( Takab , Iran ), Beshtau uranium deposit ( Caucasus , Russia ) and the Lengenbach mine in the Binn valley ( Valais , Switzerland ).
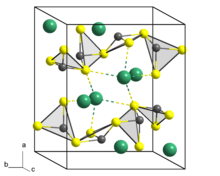
Crystal structure
Lorándite crystallizes monoclinically in the space group P 2 1 / a with the lattice parameters a = 12.27 Å , b = 11.33 Å, c = 6.11 Å and β = 104.2 ° as well as 8 formula units per unit cell .
In the crystal structure of Lorándite, each arsenic atom (As) is covalently linked to three sulfur atoms (S) d (As − S) = 2.08 - 2.32 Å), with the sulfur atoms in the base and the arsenic atom at the top of one trigonal pyramid is located as a coordination polyhedron . In consideration of the stereochemically active lone pair of As (III) cation, this arrangement can also as a ψ 1 - tetrahedra are described, wherein the As atom is in the center and the free electron pair as a pseudo-ligand in the fourth corner of an imaginary tetrahedron (see also VSEPR model ). This [AsS 3 ] 3- -Thioarsenateinheiten particles are not isolated in the structure before, but are two common S atoms to spiral chains of the Niggli formula condensed along the 2 1 - screw axis in the direction of [010] ( parallel to the crystallographic b axis).
The thallium atoms (Tl) in turn form the tip of a distorted tetragonal pyramid, in whose base there are four sulfur atoms ( d (Tl − S) = 2.95 - 3.30 Å). Further bonds to S atoms above the Tl tips of these coordination pyramids with d ' (Tl − S) = 3.36 - 3.87 Å can also be considered, which means that the Tl coordination spheres can be expanded to seven with simple capped, trigonal prisms as coordination polyhedra results. The Tl (I) cations link the individual chains of thioarsenate units with one another, creating the three-dimensional crystal structure.
use
Lorándit is of little industrial importance. In some deposits it is relevant for the extraction of thallium.
Lorándit has been of scientific interest since the end of the 20th century as a possible dosimeter for measuring solar activity. Thallium isotope 205 Tl transformed by bombardment with neutrinos in the lead isotope 205 to Pb. The determination of the 205 Pb content of old thallium minerals allows conclusions to be drawn about the intensity of the sun's neutrino radiation.
See also
literature
- Lorandite in: Anthony et al .: Handbook of Mineralogy , 1, 1990, 101 ( pdf ).
- József Krenner (1894): A lorándit, új ásványfaj , in: Matematikai és Természettudományi Értesitö , Volume 12, pp. 473–473 ( PDF 28.2 kB )
- ME Fleet: The crystal structure and bonding of lorandite, Tl 2 As 2 S 4 . In: Journal of Crystallography . 138, 1973, pp. 147-160 ( pdf ).
- Melvin S. Freedman et al .: Solar Neutrinos: Proposal for a New Test . In: Science . 193, No. 4258, 1976, pp. 1117-1119, doi : 10.1126 / science.193.4258.1117 .
- MK Pavićević: Lorandite from Allchar - A low energy solar neutrino dosimeter . In: Nuclear Instruments and Methods in Physics Research Section A . 271, No. 2, 1988, pp. 287-296, doi : 10.1016 / 0168-9002 (88) 90171-4 .
- A. Lazaru, R. Ilic, J. Skvarc, ES Kristof, T. Stafilov: Neutron induced autoradiography of some minerals from the Allchar mine . In: Radiation measurements . 31, No. 1-6, 1999, pp. 677-682, DOI: 10.1016 / S1350-4487 (99) 00170-5 .
Web links
- Mineral Atlas: Lorándite (Wiki)
- LOREX 2008: Geochemical and Physical research within the LOREX Project. University of Salzburg
Individual evidence
- ↑ Webmineral - Lorandite (English)
- ↑ a b c d A. Zemann, J. Zemann: On the knowledge of the crystal structure of Lorandite, TlAsS 2 In: Acta Crystallographica. Volume 12, 1959, pp. 1002-1006; ( American Mineralogist Crystal Structure Database - Lorandite )
- ↑ a b Mindat - Lorandite (English)
- ↑ Yves Moëlo, Emil Makovicky, Nadejda N. Mozgova, John L. Jambor, Nigel Cook, Allan Pring, Werner Paar, Ernest H. Nickel, Stephan Graeser, Sven Karup-Møller, Tonči Balic-Žunic, William G. Mumme, Filippo Vurro, Dan Topa, Luca Bindi, Klaus Bente, Masaaki Shimizu: Sulfosalt systematics: a review. Report of the sulfosalt sub-committee of the IMA Commission on Ore Mineralogy . In: European Journal of Mineralogy . tape 20 , no. 1 , 2008, p. 7–46 , doi : 10.1127 / 0935-1221 / 2008 / 0020-1778 (English, rruff.info [PDF; 485 kB ; accessed on August 3, 2020]).
- ^ Andreas Edenharter and Tjerk Peters, (1979). "Hydrothermal synthesis of Tl-containing sulfosalts", Journal of Crystallography: Vol. 150, No. 1-4, pp. 169-180. doi : 10.1524 / zkri.1979.150.1-4.169
- ↑ Find location list for Lorándite at the Mineralienatlas and at Mindat

![{\ mathrm {{} _ {\ infty} ^ {1} \ lbrace [As (III) S _ {{2/2}} ^ {e} S _ {{1/1}} ^ {t}] ^ {{ 1 -}}}} \ rbrace](https://wikimedia.org/api/rest_v1/media/math/render/svg/ef4bedab17d9bbca84e8bec9c00fa70ae648657d)