Air breathing in bony fish

Air breathing in bony fish is the uptake of atmospheric air by bony fish (Osteichthyes). All bony fish have gills for breathing in water. Air breathing developed at least 60 separate lines of bony fish. Air breathing can support or temporarily replace gill breathing. It can be performed as a mandatory (mandatory) or optional (optional). In the case of some fish, air breathing can be restricted to certain periods of life ( larval stage - juvenile form - adult form ). Some groups even have three breathing systems: gills, skin breathing and air breathing through a special organ.

Groups of fish
The majority of fish that can breathe air are freshwater fish from the tropics or from the estuaries. Most of the air-breathing fish today belong to the real bony fish (Teleostei). Other air-breathing fish are the lung fish (Dipnoi), the pike -fish (Polypteriformes) and the bone organoids (Holostei). A smaller number of air-breathing fish live in temperate zones. Some, such as the bald pike ( Alma calva ), live in waters that freeze over seasonally.
Reports of air-breathing cartilaginous fish are based on unconfirmed observations on squamous gills (Elasmobranchii).
Oxygen and water
Compared to air, water is poor in oxygen. The air at sea level contains about 30 times as much oxygen (O 2 ) as water that is completely saturated with oxygen. However, the oxygen content of the water is always below this value, it can even be considerably lower. Fish with two alternative respiratory systems have a correspondingly better possibility of oxygen uptake in oxygen-poor water than others.

With the air breathing, the energy requirement is eliminated by the gill ventilation, which in oxygen-poor water z. B. in carp can consume 13-22% of the total energy and becomes problematic with increasing oxygen shortage. For example, Oreochromis niloticus only uses 3% of the energy for gill ventilation in oxygen-rich water; if there is a lack of oxygen ( PO 2 below 35 mmHg ), this energy expenditure can increase to 20%.
The use of air breathing increases with increasing activity or with reduced availability of oxygen in the water and increased water temperature. Some bony fish that are able to breathe air also adapted to hypoxic water conditions with increased hydrogen sulfide content .
Hoplosternum littorale keeps its oxygen uptake relatively constant down to a lower partial oxygen pressure of 50 mmHg, with an air breathing rate of around one breath per hour. Between 40 and 50 mmHg, Hoplosternum littorale is dependent on air breathing and at 10 mmHg it needs four and a half breaths an hour. For him is hydrogen sulphide at a concentration of 70 uM ( micromolar ) acute life-threatening, if enough oxygen is available simultaneously in oxygen depletion and simultaneous air breathing hydrogen sulfide is life threatening but only at a concentration of 87 uM toxic .
Gills
All bony fish have functioning gills.
Obligatory air-breathing fish die even in oxygen-rich water if they are denied access to the surface, because their gills are undersized to obtain sufficient oxygen.
Fish that cannot breathe air are often able to use the oxygen-rich surface water via their gills under hypoxic conditions. In a study of 68 species from Panama, 93% of freshwater fish and 72% of marine fish showed such behavior, but only 42% of species that live in normally oxygen-rich marine waters.

Not all fish that can colonize hypoxic water spaces are able to breathe air. Thus Hoplias malabaricus ( Hoplias malabaricus ) only breathe through gills (although species of the same family predatory characins as Hoplerythrinus unitaeniatus or Erythrinus Erythrinus erythrinus erythrinus obligate air-breathers are). Nevertheless, at oxygen partial pressures between 150 and 25 mmHg, Traíra keeps its oxygen uptake largely constant after adjustment, even if it is adjusted to a different oxygen saturation. Adapted to normally oxygen-rich water, however, Traíra shows symptoms of a hypoxic coma in oxygen-poor water with oxygen partial pressure of 10 mmHg, which in fish that are already adapted to oxygen-poor water only occurs from 5 mm Hg oxygen partial pressure (the adaptation time for hypoxic conditions is e.g. B. 14 days at an oxygen partial pressure of 25 mmHg).
Air respiratory organs
Due to the diverse analog development paths , different bony fish use very different organ systems to exercise air breathing. Air-breathing fish usually have a hollow organ for taking up and resorbing the air; it can be parts of a transformed organ or an evolutionary new formation. It is richly equipped with vessels that supply fine capillaries or a lacunae system in the limiting membranes of the hollow organ. There are also surface-active substances , so-called surfactants , which also occur in the gills and lungs at the interfaces. Fish that often breathe air also have muscles that allow rhythmic contractions for repeated air exchange. Almost none of the bloodstreams of air-breathing fish is optimized for the supply of oxygen-poor blood to the air-breathing organ: the vessels coming out of the body still carry about half of the oxygen-rich blood, except for lung fish (Dipnoi).
Lungs

In lungfish (Dipnoi) and Flösselhechten (Polypteridae) to real-developed lungs .
The lung fish breathe air more effectively than other bony fish. This is also due to the fact that they are the only bony fish whose blood circulation is optimized for both gill breathing and lung breathing by supplying particularly low-oxygen blood to each of the two outer respiratory organs, which enables a high level of oxygen enrichment: the Australian lungfish (the only recent species of the order Ceratodontiformes) has a pulmonary circulation with divided atria, vertical hole in the cardiac septum , pulmonary veins, conus ventricles and a long spiral fold in the conus arteriosus (see also the heart of the bony fish ).
Also lepidosireniformes as the African lungfish ( Protopterus ) and South African lungfish ( Lepidosiren ) developed a similar pulmonary circulation with paired lungs, reduced anterior gill arches and well-developed spiral fold in Conus arteriosus.
Swim bladder
In addition, air breathing organs developed several times from the swim bladder , for example in the pike (Lepisosteidae), e.g. B. in Lepisosteus and the bald pike ( Amia calva ).
Digestive tract
In Hypostomus ( Loricariidae ) the air respiratory organ originated from the digestive tract . The stomach walls of Hypostomus plecostomus are much thinner, transparent and wrinkle-free compared to other bony fish.
Cranial chambers
Suprabranchial organ
The adult Labyrinthici ( labyrinth fish (Anabantoidei) and snakehead fish ( Channoidei)) have a suprabranchial organ. It consists of the widened epibranchial I or labyrinth , which are paired cavities in the skull above the gill chambers that are connected to them. They are abundantly lined by epithelia with a strong blood supply . Air taken in with the mouth is pressed into these cavities. These epithelial linings are supplied by the first and second branchial arch arteries from the anterior branch of the bifurcated aorta from the heart . The oxygen absorbed by the epithelia is not removed via the dorsal aorta, but via the anterior cardinal vein.
The function of the organ was also interpreted as an acoustic resonance amplifier, since z. B. in the dwarf gourami ( Colisa lalia ) no air pressure fluctuations typical for breathing could be observed. The idea that some of the lining cells of the mucous membrane originated from gill cells has been refuted for the climbing fish ( Anabas testudineus ); it is rather an analogue development.
Pharyngeal chambers
In Amphipnous cuchia (a gill slit eel), respiratory cavities are formed in the head as appendices to the pharynx .
Gill cover chambers

In mud jumpers as Periophthalmus vulgaris and mudskippers relatives (Oxudercinae) as Boleophthalmus boddaereti operculum chambers (were operculum ) is converted to additional breathing cavities. In Periophthalmus vulgaris they are particularly large and well supplied with vessels for breathing. Complex separate valves regulate inflow and outflow for these breathing chambers.
Skin breathing
Skin breathing is possible both in water and in air via mucous membranes (kept moist and well supplied with blood). The efficiency on land can be higher due to the higher oxygen partial pressure. These fish are usually characterized by incomplete scaling. Practice skin breathing z. B. the Atlantic butterfish ( Pholis gunnellus ), cod ( Gadus morhua ), the five-bearded sea tadpole ( Ciliata mustela ), some slimy fish (e.g. Blennius pholis ), flounder ( Platichthys flesus ), sole ( Solea solea ) and eel ( Anguilla) anguilla ). In the case of the Atlantic butterfish, cod and five-bearded sea burbot, skin breathing is not very efficient and is only sufficient to supply the skin areas themselves, but it relieves the circulation and gill breathing. In the case of sole, it is mainly the underside that is breathable.
Breathing air through the gills
Even the gills of some fish can be used to absorb atmospheric oxygen: the gill-slit eel Synbranchus marmoratus can ingest air with its mouth, and its gills absorb around 50% of the oxygen it contains within 12–15 minutes . During gill breathing in water, the oxygen saturation in its arteries never exceeds 50–60%. After taking in air, however, it regularly reaches almost complete oxygen saturation in the blood. The concentration of carbon dioxide (PCO 2 ) in the blood also increases, but only for a short time, because the carbon dioxide is released very quickly via the gills as soon as water rinses through the gill chambers.
Adjustments for shore leave
When going ashore or involuntarily dry, the gills become inoperative when they dry out, only breathing air can meet the oxygen demand. Parallel to air breathing, further physiological adaptations to the life possibilities outside the water can take place: for locomotion on land, keeping the body moist, excretion via mucous membranes .
Carbon dioxide release
One respiratory function is the uptake of oxygen, another is the release of carbon dioxide (CO₂). In bony fish, however, air breathing is primarily understood as the short-term intake of oxygen-containing air. The release of carbon dioxide through the gills is, in principle, considerably easier than the absorption of oxygen from the water and is therefore usually not a problem. With a brief gasp, there is usually no emission of carbon dioxide, which can increase the carbon dioxide concentration in the blood. This carbon dioxide can easily be given off in the water with the gills. But on land at the latest, mechanisms other than the gills must ensure that carbon dioxide is released. Then the carbon dioxide is usually released through the skin.
Moisture retention
The gill-slit eel Synbranchus marmoratus is extremely resistant to dry periods, it can survive out of the water for several months.
Air-breathing fish like climbing fish, eels, heteropneustes, and channa (a snakehead fish ) can spend long periods of time out of the water as long as they don't dry out. The loss of water is the limiting factor for these bony fish, which are well adapted to breathing. Eels such as Anguilla bengalensis can survive outside of the water even in dry conditions (with a relative humidity of 35–40%) for 3–5 hours, whereby they lose 20–23% of their body weight.
Insofar as urea is excreted via the mucous membranes, it in turn contributes to maintaining moisture by binding air moisture.
excretion
Outside the water, dry gills become functionless and the release of toxic metabolic products, nitrogen carriers , salts and ions for detoxification, osmoregulation or the balance of the acid-base balance via the gills are replaced by alternative excretion routes.
Most bony fish release ammonia through their gills as a nitrogen carrier .
Only a few fish have the alternative of the urea cycle and can also release small amounts of urea via their gills or mucous membranes. These include the monkfish ( Lophius piscatorius ), the cod ( Gadus morhua ), the puffer fish Sphoeroides maculatus , eels such as Anguilla rostrata , the carp ( Cyprinus carpio ), the climbing fish ( Anabas testudineus ), Sicyases sanguineus (a tortoiseshell ), the Asian small fish snakehead ( Channa gachua ) Blennius pholis (a Schleimfisch ) Mystus vittatus (a sting Wels ) Tilapiaarten from the Lake Magadi , and others who are amphibian in the intertidal zone. As long as they are surrounded by enough water, like most fish, they release nitrogen mainly through their gills in the form of ammonia. If they are no longer in the water, many of those capable of doing so primarily release urea through their mucous membranes. In some fishes decide less environmental conditions, but rather the development stage of the selection of the nitrogen carrier: Like most fish are also adult rainbow trout incapable of urea output, in contrast, juvenile rainbow trout, four of the necessary for urea production enzymes produced .
Bony fish with skin respiration can often absorb and release other substances through their mucous membrane. Like many others, the gill-slit eel Symbranchus marmoratus also absorbs or releases ions for its osmoregulation, for regulating its acid-base ratio and for nitrogen excretion. In the case of Symbranchus marmoratus , the route via the skin predominates but the route via the gills; its skin can be viewed as an ion transport organ.
Locomotion
The climbing fish ( Anabas testudineus ) moves skillfully on land, giving it its name. With its caudal fin it pushes itself off the ground and claws itself into unevenness with the spinous processes of its gill cover. Unusually for a bony fish, its gill cover bones are not connected to a fixed operculum, but rather can be moved against each other. Tired or crashed, the climbing fish can also push itself forward with its tail fin lying on its side, whereby a gill cover spike can drill into the subsoil to support it. The maximum speed is 1.8 body lengths per second, the maximum incline is 30 ° on meadow, he can overcome obstacles of at least half his body length vertically, but can hardly climb woody plants.
A particularly effective way of locomotion on land is practiced by mudskippers ( periophthalmos ) and mudskippers , which is what gives them their name. Periophthalmos can also reach higher areas and even climb mangrove roots. The pectoral fins transmit the jumping force.
Eels like the European eel ( Anguilla anguilla ) have retained their snake-like mode of locomotion on land, which they also use in the water. The work is done by the longitudinal muscles. The muscle performance and achieved speed in water is higher than on land, which is explained by the fact that the adhesion of the small contact surface on land does not allow for a higher speed during this movement.
Conduct on land
As different as the anatomical-physiological adaptations that make shore leave possible, the advantages and ways of life of the groups of bony fish equipped with them are just as varied.
nutrition
Most mudskipper relatives eat vegetable food when they go ashore, mudskippers ( periophthalmos ) also hunt insects and small crustaceans for their food.
migration
Various air-breathing fish such as climbing fish, eels, tube-gill catfish ( heteropneustes ) and channa (Asian snakehead fish ) migrate through grasslands from one body of water to another. The climbing fish is said to migrate from one body of water to another during rainy seasons to spawn there.
evolution
Accessory breathing
The most important selection pressure for the multiple parallel development of air breathing that supports gill breathing in bony fish comes from the lack of oxygen in the habitat. Air-breathing bony fish appeared in the late Silurian or early Devonian . It is believed that they evolved as adaptations to hypoxic conditions and that this ultimately evolved into pulmonary breathing in terrestrial vertebrates.
The urea synthesis pathway may have evolved in bony fish to detoxify ammonia under alkaline water conditions (< pH 9.0-9.5) .
The swim bladder was formed from an unpaired dorsal protuberance of the foregut (pharynx) of the bony fish. It represents a further development of the primary fish lung with a functional change from a respiratory organ to a hydrostatic organ. In some air-breathing bony fish, this has developed into a respiratory organ again.
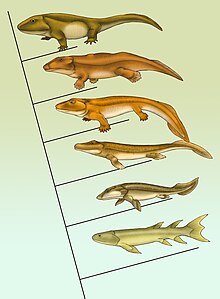
Eusthenopteron , Panderichthys , Tiktaalik , Acanthostega , Ichthyostega , Pederpes
Shore leave
It has been suggested that the lungs of terrestrial vertebrates are homologous to the swim bladder. This contradicts other findings that suggest a homology of the lungs of the lungfish (Dipnoi), the pike-pike (Polypteriformes) and the terrestrial vertebrates, to which the swim bladder is an analogous organ. The lungs of the lungfish and pike-tailed pike emerged from paired protuberances in the pharynx behind the gill chamber. The supplying vessels correspond to the artery and vein of the sixth gill arch. But despite the diverse findings, the homology relationships between the swim bladder, the lungs of the bony fish, the lungs of the terrestrial vertebrate and other air breathing systems of the bony fish have not been clarified beyond doubt, because every attempt at an explanation faces one or the other contradiction and the sequence of some developmental steps is unclear.
The circulatory system of the Australian lungfish (Neoceratodontidae) with real lung circulation is homologous to the circulatory systems of the terrestrial vertebrates.
The development of the terrestrial vertebrates proceeded via a still unknown ancestor within the bony fish with lungs and probably already quadruped ( quadruped ) locomotion. In this respect, the bony fish do not represent a monophyletic group. The skin breathing of amphibians may have been developed secondarily and does not have to be homologous to the skin breathing of a bony fish ancestor. The recent amphibians allow little conclusions to be drawn about the physiological and anatomical features of the early forms. A number of intermediate forms of "shore leave" from the Devonian are well documented through fossils.
Single receipts
- ↑ Kurt Fiedler: Air breathing in bony fish. In: Kurt Fiedler (Ed.): Textbook of Special Zoology. Volume II, Part 2: Fish. Gustav Fischer Verlag, Jena 1991, ISBN 3-334-00339-6 .
- ↑ a b c d e f g h i j M. L. Glass, FT Rantin: Gas exchange and control of respiration in air-breathing teleost fish. In: Cardio-Respiratory Control in Vertebrates . Springer, Berlin / Heidelberg 2009, ISBN 978-3-540-93984-9 , pp. 99-119. doi : 10.1007 / 978-3-540-93985-6_5
- ↑ a b Jeffrey B. Graham (Ed.): Air-breathing fishes: evolution, diversity, and adaptation. Academic Press, 1997, ISBN 0-12-294860-2 .
- ↑ a b c d e f g h i j k l m Kjell Johansen: Air breathing in fishes. In: William Stewart Ioar, David J. Randall (Eds.): Fish Physiology. Volume 4, Academic Press, 1970, pp. 361-411.
- ↑ Riaz Ahmad, Absar-ul Hasnain: Ontogenetic changes and developmental adjustments in lactate dehydrogenase isozymes of an obligate air-breathing fish Channa punctatus during deprivation of air access. In: Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology , Volume 140, No. 2, 2005, pp. 271-278.
- ↑ EG Affonso, FT Rantin: Respiratory responses of the air-breathing fish> Hoplosternum littorale to hypoxia and hydrogen sulfide. In: Comparative Biochemistry and Physiology - Part C Toxicology & Pharmacology , Vol. 141, No. 3, 2005, pp. 275-280.
- ^ Donald L. Kramer: Aquatic surface respiration in the fishes of Panama: distribution in relation to risk of hypoxia. In: Environmental Biology of Fishes , Vol. 8, No. 1, 1983, pp. 49-54.
- ↑ Francisco Tadeu Rantin, Kjell Johansen: Responses of the teleost Hoplias malabaricus to hypoxia. In: Environmental Biology of Fishes , Vol. 11, No. 3, 1984, pp. 221-228.
- ↑ a b c d e f g h Hiran M. Dutta, JS Datta Munshi: Functional morphology of air-breathing fishes: A review. In: Proceedings: Animal Sciences , Vol. 94, No. 4, 1985, pp. 359-375.
- ↑ CF Phleger, BS Saunders: Swim-bladder surfactants of Amazon air-breathing fishes. In: Canadian Journal of Zoology , Vol. 56, No. 4, 1978, pp. 946-952.
- ↑ a b c d e f Warren W. Burggren, Kjell Johansen: Circulation and respiration in lungfishes (Dipnoi). In: Journal of Morphology , Vol. 190, No. S1, 1986, pp. 217-236. doi : 10.1002 / jmor.1051900415
- ↑ Dagmara Podkowa, Lucyna Goniakowska ‐ Witalińska: Morphology of the air ‐ breathing stomach of the catfish Hypostomus plecostomus. In: Journal of Morphology , Vol. 257, No. 2, 2003, pp. 147-163, doi : 10.1002 / jmor.10102 .
- ↑ a b c d e f Wilfried Westheide, Gunde Rieger: Labyrinthici (Anabantoidei and Channoidei). In: Wilfried Westheide, Gunde Rieger (Hrsgb.): Special Zoology. Part 2: Vertebrate or skull animals, Part 2 , Life Sciences, Springer, 2010, Chapter 5.15.4.14, p. 305
- ^ W. Westheide, G. Rieger (Ed.): Special Zoology. Spektrum Akademischer Verlag, Heidelberg 2004, ISBN 3-8274-0307-3
- ↑ Stefan Schuster: The volume of air within the swimbladder and breathing cavities of the anabantoid fish Colisa laua (Perciformes, Belonthdae). In: Journal of Experimental Biology , Vol. 144, No. 1, 1989, pp. 185-198.
- ↑ a b c G. Nonnotte, R. Kirsch: Cutaneous respiration in seven sea-water teleosts. In: Respiration Physiology , Vol. 35, No. 2, 1978, pp. 111-118.
- ↑ a b c Kjell Johansen: Air breathing in the teleost Symbranchus marmoratus. In: Comparative Biochemistry and Physiology , Vol. 18, No. 2, 1966, pp. 383-395.
- ↑ a b c d Gordon R. Ultsch: The potential role of hypercarbia in the transition from water-breathing to air-breathing in vertebrates. In: Evolution , Vol. 41, No. 2, 1987, pp. 442-445. doi : 10.2307 / 2409152
- ↑ a b c d e D. Mallikaraj et al .: The survival out of water and evaporative water loss in Anguilla bengalensis related to relative humidity. In: International Journal of Pharmaceutical & Biological Archive , Volume 2, No. 2, 2011.
- ^ SL Hora: Physiology, bionomics and evolution of the air-breathing fishes of India. In: Trans. Nat. Inst. Sci. , India, Vol. 1, pp. 1-16.
- ↑ a b c d e Homer W. Smith: The excretion of ammonia and urea by the gills of fish. In: Journal of Biological Chemistry , Vol. 81, No. 3, 1929, pp. 727-742.
- ↑ a b c d M. Ramaswamy, T. Gopalakrishna Reddy: Ammonia and urea excretion in three species of air-breathing fish subjected to aerial exposure. In: Proceedings: Animal Sciences , Vol. 92, No. 4, 1983, pp. 293-297. doi : 10.1007 / BF03186197
- ↑ J. Cancino, J. Castilla: Emersion behavior and foraging ecology of the common Chilean clingfish Sicyases sanguineus (Pisces: Gobiesocidae). In: Journal of Natural History , Volume 22, 1988, pp. 249-261.
- ↑ J. Davenport, MDJ Sayer: Ammonia and urea excretion in the amphibious teleost Blennius pholis (L.) in sea-water and in air. In: Comparative Biochemistry and Physiology Part A: Physiology , Vol. 84, No. 1, 1986, pp. 189-194.
- ↑ a b c Patricia A. Wright, Michelle D. Land: Urea production and transport in teleost fishes. In: Comparative Biochemistry and Physiology-Part A: Molecular & Integrative Physiology , Volume 119, No. 1, 1998, pp. 47-54.
- ^ Norbert Heisler: Intracellular and extracellular acid-base regulation in the tropical fresh-water teleost fish Synbranchus marmoratus in response to the transition from water breathing to air breathing. In: Journal of Experimental Biology , Vol. 99, No. 1, 1982, pp. 9-28.
- ^ Daniel F. Stiffler et al .: Cutaneous ion transport in the freshwater teleost Synbranchus marmoratus. In: Physiological Zoology , 1986, pp. 406-418.
- ^ J. Davenport, AKM Matin: Terrestrial locomotion in the climbing perch, Anabas testudineus (Bloch) (Anabantidea, Pisces). In: Journal of Fish Biology , Volume 37, No. 1, 1990, pp. 175-184, doi : 10.1111 / j.1095-8649.1990.tb05938.x .
- ↑ Vernon A. Harris: On the locomotion of the mud ‐ skipper Periophthalmus koelreuteri (Pallas) :( Gobiidae). In: Proceedings of the Zoological Society of London , Volume 134, No. 1, 1960. doi : 10.1111 / j.1469-7998.1960.tb05921.x
- Jump up ↑ DJ Ellerby, ILY Spierts, JD Altringham: Fast muscle function in the European eel (Anguilla anguilla L.) during aquatic and terrestrial locomotion. In: Journal of Experimental Biology , Vol. 204, No. 13, 2001, pp. 2231-2238.
- ↑ B. K: Das: The bionomics of certain air-breathing fishes of India, together with an account of the development of their air-breathing organs. In: Phli. Trans. Ser. B. , Vol. 216, No. 433, 1927, pp. 183-219.
- ^ GM Natarajan: Studies on the respiration of Anabas scandens (Cuvier). M.Sc. thesis submitted to ICAR, New Delhi, India, 1972.
- ^ Joseph Barrell: The Influence of Silurian-Devonian Climates on the Rise of Air-Breathing Vertebrates. In: Proceedings of the National Academy of Sciences , Volume 2, No. 8, 1916, p. 499. PMC 1091077 (free full text)
- ^ A b Colleen Farmer: Did lungs and the intracardiac shunt evolve to oxygenate the heart in vertebrates. In: Paleobiology , Vol. 23, No. 3, 1997, pp. 358-372.
- ↑ a b c d e f Steven F. Perry et al .: Which came first, the lung or the breath? In: Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology , Vol. 129, No. 1, 2001, pp. 37-47.
- ^ Freye, Hans-Albrecht: Zoologie , 9th edition. Fischer Verlag, Jena 1991, ISBN 3-334-00235-7
- ↑ a b c Alfred Sherwood Romer: Skin breathing - Primary or secondary? In: Respiration Physiology , Vol. 14, No. 1, 1972, pp. 183-192.
- ↑ Till Biskup: Tribal History of the Vertebrates I: Fish and Amphibians. ( Memento of the original from July 10, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Dump from December 11, 2012, PDF
- ↑ Myths and Evolution. Handout for teachers with working materials ( memento of the original from March 23, 2011 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , Universum Bremen, dump of December 11, 2012, PDF
- ^ Matthias Glaubrecht: How amphibians once went ashore. In: Die Welt from June 27, 1997, dump from December 11, 2012
- ^ Donald Prothero: What missing link? Reports of huge gaps in the fossil record have been greatly exaggerated. In: New Scientist . No. 2645 of March 1, 2008, pp. 35-41. ISSN 0262-4079 , short version , doi : 10.1016 / S0262-4079 (08) 60548-5

