Mobile drinking water production

Portable, mobile or relocatable water treatment systems ( portable water purification, point-of-use ( POU ) water treatment systems, field water disinfection ) are independent, self-contained units that are used for the treatment of drinking water from raw water that comes from untreated sources, such as Rivers, lakes or near-surface groundwater are suitable. The aim of these systems, and also devices for smaller amounts of water, is the portable supply of low-germ drinking water that is free of toxic substances. The mobile water treatment is used both in the event of a disaster and in the leisure or military sector.
In accessible, densely populated areas, larger container systems that use reverse osmosis , UV or filter systems for processing can be used. The use of mobile truck container systems is also common. If there is less water required in areas that are difficult to access or sparsely populated, it is also possible to use water purification tablets or SODIS (water treatment in PET bottles using solar radiation). Mountaineers, campers and travelers also use many types of small portable water treatment systems in remote areas to meet their own needs. The water requirement for light physical activity and 43 ° C is around 1.3 liters per hour through sweating . Since water has a high density , it is not always possible to carry sufficient amounts of clean drinking water with you, for example when trekking in the desert. If necessary, chemical additives can also be used to treat germs. Complete solutions also exist for the home and house, for example to remove chlorine , chloramines , undissolved corrosion particles, for example rust from the pipe network, heavy metals or unpleasant odors from the water provided by the local water supplier and to improve the taste.
Types of water pollution and their consequences

Rivers can be polluted by sewage, land pollution and subsequent wash-in by rain and industrial sewage - even from far upstream.
Even small rivulets, springs and wells can be contaminated with animal remains and remains and consequently with pathogens . The presence of dead animals upstream is not uncommon. Almost everywhere, the water can be contaminated with bacteria , protozoa and parasites (e.g. worms or their other stages from human or animal feces ). Roots penetrating into spring areas can also cause contaminated surface water to enter. Pathogenic strains of Escherichia coli , an intestinal bacterium, survive - even if only for a short time - outside the (human) body and can thus infect new "hosts". Infection with EHEC or Shigella dysenteriae , as has often been observed in the past, can lead to bloody diarrhea.
Giardia lamblia and Cryptosporidium spp. ( Protozoa ) both cause diarrhea ( giardiasis , cryptosporidiosis ) and also occur in temperate latitudes. In tropical regions, for example in Hawaii, you also have to deal with Leptospira spp. (Bacterium) count. The protozoa Cyclospora (pathogencausing "traveler'sdiarrhea", especially thespecies Cyclospora cayetanensis thatcan becombatedwith cotrimoxazole or ciprofloxacin ), Balantidium coli (can cause large intestinal ulcers) and Isospora belli (diarrhea, treatment with cotrimoxazole is possible if necessary) spread.
Vibrio cholerae , Vibrio eltor (pathogen causing cholera ) and Salmonella strains , which can cause typhoid and the weaker form, paratyphoid , are less common in industrialized countries . Pathogenic viruses are found next to the metacercariae of the great liver fluke ( Fasciola hepatica ), which are particularly common in areas frequented by sheep, game or cattle. Plants growing near water (e.g. watercress ) could also be covered with encysted metacercariae.
The immune system protects healthy adults from bacterial diseases better than children and old people. The ingestion of a few thousand typhoid bacteria rarely leads to disease. The danger here lies in processing it into food, which then serves as a breeding ground. A few viruses or cysts are often enough to trigger viral diseases and parasite infestation.

Processing method
Boil
Boiling water kills bacteria and microorganisms such as the protozoa mentioned above. At water temperatures above 70 ° C, pathogenic germs are killed within 30 minutes , at 85 ° C within a few minutes and at 100 ° C most of them, with the exception of a few spores and toxins (e.g. those of Clostridium botulinum ), are killed. Since the boiling point of the water drops at high altitudes and therefore does not reach 100 ° C, you have to cook longer in order to have the same success. In contrast, higher boiling water temperatures than normal atmospheric pressure can be achieved with pressure cookers and therefore boiling can be carried out more quickly.
It should also be noted that a lot of soiling, for example almost all inorganic ingredients (for example heavy metal salts , colloidally distributed metals and their oxides) and toxic non-volatile organic substances cannot be removed by boiling. These can largely be eliminated by flocculation and subsequent filtering. Many poisonous organic contaminants can be removed with the procedure described under activated carbon filter .
Filtration
Portable filter pumps with ceramic filters, which deliver 5000 to 50,000 liters per filter cartridge and filter out pathogens as small as 0.2 to 0.3 µm, are commercially available. Some also use filters with activated carbon for cleaning. Most systems of this type remove almost all bacteria and protozoa, but not viruses. Therefore, disinfection with germicidal chemicals or UV light is also required. Furthermore, not all thread-like bacteria (e.g. Leptospira spp. , Diameter about 0.1 µm, which trigger leptospirosis ) are separated from purely mechanical systems such as the pencil-thin LifeStraw with the 0.2 µm filter . In order to compensate for technical inadequacies and other problems, chemicals such as chlorine, chlorine dioxide , silver chloride , iodine and sodium hypochlorite should also be added to the treated water.
In the past, polymer and ceramic filters that contained a built-in source of iodine were often used for post-treatment. Most of these filters are no longer offered and used because of the unpleasant taste of the water and the harmful effects of iodine on long-term use and certain previous illnesses.
The filters work great when new, removing a lot of bacteria and fungus. However, there is a risk that they will be attacked by microorganisms themselves. In recent years, therefore, ceramic elements and / or activated carbon treated with silver nanoparticles have been used in particular to prevent the growth of pathogens. If there is a higher content of undissolved and colloidal impurities in the raw water, there is a risk that they will silt up due to these substances being filtered off. This can cause the filter resistance to rise too quickly and the amount of pure water produced to drop to an inadmissible level. Only systems with backwashable filters are less affected by this problem.
Larger container systems mainly have the following system components for filtering:
- Raw water pump (s)
- a flocculant
- a filter device consisting of a fine filter (made of ceramic or plastic) or a backwashable filter with gravel and / or activated carbon or a precoat filter
- a dosing station for adding a chemical to disinfect the pure water
Membrane process
The membrane technology is suitable for various mobile water treatment systems, as it can be built up modularly and can be adapted to the relevant conditions by selecting the pore size.
Ultrafiltration
There are already portable ultrafiltration units that can be easily brought into disaster areas and treat water for up to 400 people per day without the need for electricity. One example is the “ water backpack ”.
Reverse osmosis
Small, hand-operated reverse osmosis systems were originally developed for military use in the late 1980s and were integrated into inflatables on board aircraft as survival equipment, for example. Now there are also devices for the civil sector. The pump function is similar to that of a grease gun . This allows drinking water to be obtained from salt water . To prevent contamination, these devices should be disinfected at regular intervals. In order to achieve a sufficient rate of desalination, 55 bar is usually applied to salt water. The necessary effort is correspondingly high and even the production of small amounts of water is laborious. Since the devices have a pump mechanism, an ultra-fine, semipermeable membrane and have to withstand high pressures and thus the effects of forces, they are quite expensive compared to other processing mechanisms.
Adsorption on activated carbon
Activated charcoal has a large surface area and adsorbs many dissolved and colloidal compounds. It should be noted that in addition to toxic organic compounds for human consumption, a large number of non-toxic organic compounds are also absorbed. As a result, the absorption capacity can be exhausted too quickly. Activated charcoal is mainly used to remove organic compounds that are highly disturbing to taste and / or smell. Because of the good filtering effect for colloidal and undissolved substances, it is usually only used after another upstream filter device.
Activated carbon is therefore normally only used in addition for mobile systems, except when improving taste and smell is the main goal. The latter is predominantly the case with household filters, for example . In particular, chloramines , which are formed in chlorinated water and significantly impair the taste of the water, are largely removed by such filters.
Ion exchanger
Ion exchangers are able to replace certain anions and cations (anion exchanger, cation exchanger, mixed bed process = both) in the water with other ions. Really dissolved non-ionic organic and inorganic substances pass through the ion exchanger unhindered. Suspended particles and undissolved particles quickly lead to clogging. Since the ion exchangers - depending on the type - have to be regenerated with caustic soda , hydrochloric acid or a saline solution, ion exchange filters are only used for mobile systems in special cases. When using such filters, however, replaceable ion exchange fillings are often used. This avoids the handling of the not harmless regeneration chemicals, strongly corrosive acids and bases, on site.
In principle, only particularly pure ion exchange resins may be used for the treatment of drinking water. The release of organic components is limited to max. 3.0 mg / l limited.
For areas in which the drinking water contains high levels of carbonate hardness , the water quality can be improved with special household filters. These contain regenerated weakly acidic cation exchange resin and also activated carbon. With these filters, the water can be partially desalinated and its taste and smell can also be improved.
Chemical water disinfection
"It should be noted that disinfection is never effected by adding disinfectants alone, but always in combination with a [different] treatment measure." Therefore, the mere chlorination of water is not, but the filtration to remove the turbidity and subsequent chlorination is one Water treatment measure.
Iodine based solutions

The effects of iodine are based on the precipitation (precipitation) of proteins , adsorption and penetration. For many germs this happens in a similar period of time. Iodine should not be dissolved in potassium iodide, otherwise the effectiveness decreases due to the formation of triiodide and periodide. The pH value should be low, the temperature high and the pollution with organic substances low in order to ensure optimal effectiveness.
Iodine tablets
When iodine is used for water treatment, it is released in the form of a solution, in crystallized form or through the tetraglycine hydroiodide contained in water purifying tablets. A water purification tablet releases 8 mg of iodine when dissolved . The iodine kills many - but not all germs from natural freshwater sources. It is an imperfect but simple way of sterilizing water. After the iodine tablet, a second tablet is usually dissolved, which consists of ascorbic acid (vitamin C) and acts as a reducing agent, thereby converting any iodine into tasteless iodide. The order is also important here; First the iodine tablet must be used and the iodine must have enough exposure time (approx. 30 min . for warm, clear water;> 30 min . for cloudy, cold water) to obtain safe drinking water. Taking iodine via tetraglycine hydroiodide tablets also lowers the absorption of radioactive isotopes of iodine , such as is released after reactor accidents, for example. However, not everyone can tolerate iodine. In the case of certain allergies, diseases and situations (non-immunogenic hyperthyroidism and pregnancy), its use is therefore contraindicated. Anyone who knows that they cannot tolerate iodine-containing contrast media, which are used in X-ray examinations, must switch to another method of sterilization. Tetraglycine hydroiodide tablets - tightly closed - have a long shelf life, although some manufacturers advise against using them 3 months after first opening.

Elemental iodine
A cheaper alternative is the use of iodine crystals (on the US market e.g. under the name Polar Pure). A small amount of water is poured into a glass bottle containing the iodine crystals for 30 minutes . serviced ("disinfectant solution") and only filled as much as needed in a larger amount of untreated water (canteen or similar). After a short waiting period - and a longer one for cold water treatment - the water in the canteen can be used as drinking water. The advantage of this method lies in the large quantities (approx. 5600 l ) of drinking water that can be produced with the help of this method from a small bottle containing iodine crystals. Pure iodine has a longer shelf life than tetraglycine hydroiodide if it is always tightly sealed and kept under water. Iodine sublimates in air . Caution should be exercised when swallowing iodine crystals and freezing the bottle in cold environments.
Chlorine based solutions

The microbicidal effect of chlorine and its derivatives is due to its reaction with cell proteins (of a structural and enzymatic nature) and nucleic acids . As a result, it has a broad spectrum of activity that encompasses all microorganisms, but spores are killed much more slowly than metabolically active (vegetative) germs. The usual chlorine concentration for disinfecting drinking water in Germany is a maximum of 0.3 mg / l and, if necessary, temporarily 0.6 mg / l. In contrast, a much higher concentration is usually required for mobile drinking water production. This is, for example, 4 ppm for light pollution, 10 ppm for medium pollution, 20 ppm of free chlorine ( ppm av.cl ) for visibly polluted water . When chlorine is dissolved or released, disproportionation creates hypochlorite ions:
- Cl 2 + H 2 O (starting materials) ↔ HClO + HCl (products)
- Chlorine + water ↔ hypochlorous acid + hydrochloric acid
If the hydrochloric acid is bound, the chemical equilibrium can shift to the side of the products and a lot of hypochlorite is created, which is a strong oxidizing agent. Other factors that promote a successful disinfection are a low pH value, low chlorine consumption (reaction of chlorine with other substances or adsorption), high temperature and a high redox potential (depending on the pH value). Chlorine is three times more effective against Escherichia coli than an iodine solution of the same concentration. However, even chlorine concentrations of 10 ppm are not able to kill all germs: Mycobacterium tuberculosis ( tuberculosis pathogens , here 50 ppm would be necessary) and the fungi Aspergillus niger and Rhodotorula flava survive . For hepatitis viruses, 30 min. at 3.25 ppm at pH 6.8.
Neither chlorine nor iodine are considered fully effective against Cryptosporidium , although they show some effectiveness against Giardia intestinalis . The iodine solution must be used for at least 30 min. act on the water to be treated. Better equipment also includes a 0.2 µm ceramic pump filter insert, which is upstream of the chemical disinfection and removes protozoa, bacteria and large viruses. This combination is more effective than UV treatment even with slightly cloudy water, as this requires clear water.
Halazon tablets
Halazone tablets (p- (N, N-dichlorosulfamyl) benzoic acid, "Halazone" ) were often used in the past. Often used by US Army soldiers in World War II and included in accessory packages for one-man packaging until 1945 , the main problem was the shelf life (≤ 3 days) of opened bottles. One tablet contains 4 mg halazone, which is stabilized with sodium chloride and soda ( sodium carbonate ). When disinfecting 1 l of water, a concentration of 4 ppm is established, which E. coli , Salmonella typhi and Vibrio comma within 30 min. kills.
Sodium dichloroisocyanurate

Sodium dichloroisocyanurate (NaDCC), the sodium salt of dichloroisocyanuric acid , has largely pushed halazone tablets from the market. NaDCC is pressed with an effervescent powder (here: adipic acid and sodium hydrogen carbonate ) to improve the rate of dissolution. The decomposition in water produces hypochlorous acid (effective) and cyanuric acid .
- Na (Cl 2 Icy) + 2 H 2 O → 2 HClO + 2 H + + NaIcy 2−
Bleached chlorine tablets are a more stable basis for water disinfection than liquid bleaching agents ( sodium hypochlorite ), as the effect of the liquid bleaching agents is impaired over time and these gave contradicting results in tests and these can therefore be classified as too unreliable for the layperson. Regardless of the fact that chlorine-based mobile water treatment in tablet form, like the halazon tablets, has largely been replaced, chlorine-containing bleaches can be used for short-term emergency water supply. Two drops of unscented, 5% bleach can be added per liter and then left covered for 30 to 60 minutes. After this treatment, the cover is removed and the water left open to minimize chlorine odor and taste. The organizations Centers for Disease Control & Prevention (CDC) and Population Services International (PSI) carrying a comparable product (a 0.5 to 1.5% sodium hypochlorite) under its Water Security Strategy ( Engl. Safe Water System (SWS) ). The products are sold and distributed under various names in developing countries.
Chlorite-acid process

In addition, the chlorite-acid method is particularly interesting in distressed areas: For this purpose, 1.6 mg Natriumchlori t and 1.5 mg of sulfamic acid added to one liter of water. This easy-to-use mixture releases 1 ppm of chlorine through a chemical reaction.
- 5 NaClO 2 + 4 HSO 3 NH 2 → 4 ClO 2 + 4 NaSO 3 + NaCl + 2 H 2 O
- Sodium chlorite chlorine dioxide + sulfamic acid → + Natriumamidosulfonat (or sulfamate) + Natriumchlori d + water
Chlorine dioxide has the advantage of removing bad odors from water containing phenol; Amidosulfonates reduce the odor, taste and irritation of the hypochlorite produced by a further reaction.
Silver based solutions
In certain cases, an alternative is to use silver ion and chlorine dioxide tablets, which are sold under brand names such as Micropur Forte , Aquamira and Pristine . When used properly, they leave little taste and kill Cryptosporidium and Giardia . The main disadvantage is the long exposure time, which is normally 30 minutes. up to 4 hours. The silver ions remaining in the water improve the shelf life, but are not suitable for water disinfection on their own. This procedure should not be used chronically, otherwise silver can deposit and accumulate in certain body tissues, which leads to the rare disease Argyria . The chlorine dioxide treatment is around four times more expensive than the iodine treatment.
Peroxides - Oxygen Based Solutions
Many peroxides cannot be used for mobile water disinfection because of their complicated handling or their poor effect. Sodium perborate can form peracetic acid with an activating substance . It is used in mouthwashes and mouthwashes. Therefore one can at least assume a bacteriostatic effect.
UV light exposure
UV light induces the development of covalent crosslinks (crosslinks) on DNA ( thymine ) and RNA ( uracil ) and thus prevents the multiplication of microbes, which thereby become less harmful. Germicidal UV-C radiation (100–280 nm ) is absorbed by thymine (through its aromatic structural component) . With neighboring molecules on the DNA helix, a covalent bond is then formed between the two. As a result, the DNA can no longer be read at these points and translated into mRNA , which in the case of protein-coding genes means that the protein can no longer be produced.
UV water conditioners are also used - mostly to kill algae - in small ponds in the garden and in aquariums. The advantages are the durability and compactness of the devices. The disadvantage is the power consumption and the need for clear water without sediments. Also, some microbes are still alive, and when the water is exposed to visible light, repair enzymes ( photolyases ) begin to repair the microorganisms' DNA. Therefore, UV-treated water must not be exposed to light unless you plan to use it immediately.
Another problem is a certain resistance of some pathogens to UV radiation. It used to be assumed that cysts of protozoa were least affected by UV radiation, but this has been refuted by a more recent study. Only a dose of 6 mJ / cm² was necessary. In contrast, viruses are now said to pose the problem due to the 10 to 30 times higher radiation dose requirement. For this reason, previous filtration - especially to remove colorants - is suggested.
Water disinfection through sunlight
SODIS (abbreviation for Solar Water Disinfection ) is a method for water disinfection and is based on the germicidal effect of UV-A radiation (indirect DNA damage) in sunlight. The microbes are eliminated by temperature and UV-A light (wavelength 320–400 nm) inside colorless PET plastic bottles . For this purpose, the water in the bottles is enriched with oxygen by shaking them - incompletely filled. Then it is filled up and the bottles are exposed to direct sunlight, for which corrugated iron roofs facing the sun (roll-away protection) are well suited. Six hours in direct sunlight or two days in inconsistent weather with sun are enough to kill almost all possible microbes. This process enables inexpensive drinking water production in developing countries and in exceptional situations. Glass bottles cannot be used because they absorb strongly in the UV range .
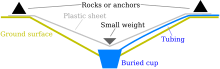
Distillation by solar radiation
Solar distillation can be done in prefabricated solar distillation equipment or as a makeshift with the help of often available components that are deposited in a recess dug in the ground. Radiated solar energy heats the water until it evaporates. The water vapor normally condenses on a plastic film that is hung up as an upside-down cone and drips into a collecting container that is attached below the center. In order to achieve a continuous operation, the water is led out of the container with a hose. Frequent opening of the apparatus, in which the water vapor atmosphere under the cover is lost, is no longer necessary. It is also possible to put a plastic bag over an overgrown patch and use it to catch the plants breathing water. This method, even if many of the same materials are used, should not be confused with SODIS. In extreme cases, distillation can even be used to produce usable drinking water from salt water or your own urine. Due to the large amount of heat of evaporation required and the high specific heat capacity of the water, this safe method is quite inefficient. Here is an example calculation: The question: "How much water can evaporate per unit of time and thus ideally be obtained."
| Example calculation: legend | ||
|---|---|---|
| Solar radiation strength , angle of incidence, specific heat of vaporization of water, specific heat capacity of water, assumed temperature difference : current temperature to boiling temperature, diameter of the system, solar power , time , energy , heat | ||
| Sample values | ||
| No. | Derivation | invoice |
| (1) | ||
| (2) | ||
| (3) | ||
| (4) | ||
| (5) | ||
| (6) | Unit consideration | |
| (7) | ||
| (8th) | ||
| (9) | ||
| (10) | ||
As the calculation shows, the recovery of 300 ml of water under ideal conditions that are never achieved, assuming 1 g = 1 ml of water, (300 g / (0.1 g / s) = 3000 s = 50 min) takes almost one Hour. In practice, much longer waiting times due to losses (heat loss to the environment, escape of water vapor, reflection of solar radiation) can be expected. Also evaporation is not included here.
Self-made water filters
Water filters can be built in place from available materials such as grass, charcoal, and sand. This is what soldiers and "nature lovers" used to do. Based on their simplicity, they can also be used by the poor, who usually have no access to safe drinking water. Unfortunately, these filters can only reduce the number of pathogens or other harmful ingredients, if at all, and thus give a deceptive feeling of security.
Pressed juices
In principle it is also possible to use plants for the extraction of low-germ water. However, the amounts contained are too small to meet the water demand, especially in desert regions. In addition, the use of unknown plants can lead to vomiting and diarrhea so that more water is lost than is absorbed.
Comparison of the procedures
| Water purification process | Biological hazards | Chemical hazards | Smell & taste | |||
| Bacteria & spores | Worms & Protozoa | Viruses | organ. links | Heavy metals | ||
| Preparation by boiling |
|
|
|
|
|
|
| Microfiltration with 0.5 µm pore diameter |
|
|
|
|
|
|
| Processing with reverse osmosis |
|
|
|
|
|
|
| Treatment with activated carbon |
|
|
|
|
|
|
| Chemical water treatment |
|
|
|
|
|
|
| Water disinfection using sunlight or UV light |
|
|
|
|
|
|
| Distillation by solar radiation |
|
|
|
|
|
|
| Self-made "water filters" |
|
|
|
|
|
|
|
|
||||||
literature
- Mark W LeChevallier and Kwok-Keung Au (2004) Water Treatment and Pathogen Control (PDF; 1.1 MB). World Health Organization. Retrieved April 13, 2010
- Franz-Bernd Frechen: Construction of a simple membrane filtration device (prototype) for the treatment of drinking water from surface water for small groups of people in emergency situations without external energy , free printable version (PDF; 13.4 MB)
Web links
- Deutschlandfunk (radio): transportable water treatment
- (Engl.) US Environmental Protection Agency (EPA): Water filters in comparison (PDF; 15 kB)
swell
- ↑ a b c Sven Siebrand: Falling offside in no man's land . (Referring to the lecture Wilderness Medicine: Medical aspects of desert trekking by Dr. Fritz Holst) Pharmazeutische Zeitung of June 13, 2013, p. 2152 ff. (In the issue on p. 42 ff.) ISSN 0031-7136 online edition .
- ^ A b c d Karl Höll: Water - use in the cycle, hygiene, analysis and evaluation. Andreas Grohmann (ed.). 8th edition, 2002, Walter de Gruyter; Berlin, New York, p. 288 ff .; ISBN 3-11-012931-0 .
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 291.
- ↑ Marianne Abele-Horn (2009), p. 292.
- ↑ Katadyn Survivor 35 - A Hand-Operated Reverse Osmosis System ( Memento February 14, 2012 in the Internet Archive ), accessed February 25, 2011.
- ↑ a b Katadyn Survivor 06 - Operating Instructions ( Memento from February 15, 2010 in the Internet Archive ) (PDF; 161 kB).
- ^ Lurgi Information; In: T 1158 /2.81, point 4.1 Drinking water treatment.
- ↑ FF Becker, D. Stetter, U. Janowsky and H. Overath; In: Breakdown of monochloramines by activated carbon filters ; gwt Wasser Abwasser, 131st year 1990, No. 1, pp. 1-8.
- ↑ e.g. in Germany: Federal Law Gazette 28 No. 1 1985; For: Health assessment of plastics in the context of the food and consumer goods law .
- ^ A b Karl Höll: Water - use in the cycle, hygiene, analysis and evaluation. Andreas Grohmann (ed.). 8th edition, 2002, Walter de Gruyter; Berlin, New York, p. 619 ff .; ISBN 3-11-012931-0 .
- ↑ HJ LeMar, WJ Georgitis, MT McDermott: Thyroid adaptation to chronic tetraglycine hydroperiodide water purification tablet use. In: The Journal of clinical endocrinology and metabolism. Volume 80, Number 1, January 1995, pp. 220-223. doi: 10.1210 / jcem.80.1.7829615 . PMID 7829615 .
- ↑ Mutschler drug effects, 9th edition, Wissenschaftl. Verlagsgesellschaft mbH Stuttgart, pp. 390, 102, ISBN 978-3-8047-1952-1 .
- ↑ (en) Repackaging Potable Aqua .
- ^ A b c d e f g h i Wallhäußer, Karl H .: Practice of Sterilization, Disinfection, p. 631 ff., 5th edition 1995, Georg Thieme Verlag, Stuttgart; ISBN 3-13-416305-5 .
- ↑ A. Grohmann (Ed.): The Drinking Water Ordinance - Introduction and Explanations for Water Supply Companies and Monitoring Authorities, p. 579, 4th edition, 2003, Erich Schmidt Verlag, Berlin; ISBN 978-3-503-05805-1 .
- ↑ Koski TA, Stuart LS, Ortenzio LF: Comparison of chlorine, bromine, iodine as disinfectants for swimming pool water . In: Applied Microbiology . 14, No. 2, March 1966, pp. 276-9. PMID 4959984 . PMC 546668 (free full text).
- ↑ CDC: SWS , PSI: SWS ( Memento of March 6, 2012 in the Internet Archive )
- ↑ Qiu X, Sundin GW, Chai B, Tiedje JM: Survival of Shewanella oneidensis MR-1 after UV radiation exposure . In: Applied and Environmental Microbiology . 70, No. 11, November 2004, pp. 6435-43. doi : 10.1128 / AEM.70.11.6435-6443.2004 . PMID 15528503 . PMC 525172 (free full text).
- ↑ USEPA, Ultraviolet Disinfection Guidance Manual for the final LT2ESWTR, Nov 2006
- ↑ National Primary Drinking Water Regulations: Long Term 2 Enhanced Surface Water Treatment Rule . In: US Environmental Protection Agency (Ed.): Federal Register . 71, No. 3, January 5, 2006, p. 783. Retrieved April 17, 2010.
- ↑ AA Mofidi, Meyer EA, Wallis PM, Chou CL, Meyer BP, Ramalinham S, Coffey BM: The effect of UV light on the inactivation of Giardia lamblia and Giardia muris cysts as determined by animal infectivity assay . In: Water Research . 36, No. 8, April 2002.
- ↑ (en) www.woodcraftwanderings.org about water purification, accessed on March 21, 2013 .
- ↑ a b Franz-Bernd Frechen: Construction of a simple membrane filtration device (prototype) for the treatment of drinking water from surface water for small groups of people in emergency situations without external energy, p. 22, accessed on February 10, 2011 , free printable version (PDF; 13.4 MB )
- ↑ Mutschler: drug effects , 9th edition, Wissenschaftl. Verlagsgesellschaft mbH Stuttgart, p. 1009, ISBN 978-3-8047-1952-1 .






























![{\ displaystyle {\ frac {[m (\ mathrm {H_ {2} O})]} {[t]}} = {\ frac {[S_ {0} \ cdot \ sin (\ beta) \ cdot A] } {[c (\ mathrm {H_ {2} O}) \ cdot \ Delta T + q _ {\ text {v}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/55e8a553c4b48a15cdf9c259a107418341fd6cf4)






