Quaternary ammonium compounds
 Alkylammonium compound (cation). The corresponding anion is not shown. |
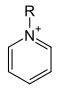 Pyridinium compound (cation). The corresponding anion is not shown. |
Quaternary ammonium compounds , sometimes QAC , quats or incorrectly quaternary ammonium compounds mentioned are organic ammonium compounds in which all four valences of a nitrogen atom organically bound. They are therefore salts ( ionic compounds), consisting of a cation and an anion . There is the amine type NR 4 + X - , in which all four R are organic radicals , and the imine type R = NR 2 + X - ; where X - is the associated anion. Also N - alkylated heteroaromatics are among the quaternary ammonium compounds.
Manufacturing
Quaternary ammonium compounds are made by reacting amines with alkylating agents , such as. B. methyl chloride , benzyl chloride , dimethyl sulfate , dodecyl bromide or ethylene oxide produced in excess.
This type of alkylation is called exhaustive alkylation .
Tertiary amines are easily converted into a quaternary amine when heated with alkyl halides. The reaction proceeds according to the following equation:
There are three different types of QAV and their subgroups, namely:
- linear alkylammonium compounds
- Alkyl trimethyl ammonium salts, e.g. B. Cetyltrimethylammonium bromide
- Dialkyldimethylammonium salts
- Benzalkonium salts, e.g. B. benzalkonium chloride
- Esterquats
- Ethoxylated QAV
- organo bentonite
- Imidazolium compounds
- Pyridinium compounds
properties
Quaternary ammonium compounds are solid ionic products. They are readily soluble in water; in contrast, insoluble in many organic solvents such as diethyl ether . In contrast to the hydrohalides of the amines, they do not form a free amine with alkali, but a stable quaternary ammonium hydroxide. This reaction is an equilibrium reaction that is very strongly shifted to the hydroxide side. The quaternary ammonium hydroxides are strong bases, comparable to sodium hydroxide and potassium hydroxide .
Quaternary ammonium compounds are thermally unstable and difficult to melt; on heating they decompose into the tertiary amine and the alkyl halide. For example, the tetramethylammonium hydroxide dissolved in water decomposes during evaporation to trimethylamine and methanol according to the reaction equation:
This slight thermal fission was noticed by August Wilhelm von Hofmann as early as 1851 and used for two generally applicable working methods:
The first method was used to determine the structure of amines. For this purpose, all hydrogen atoms of the amino group of the amines in question were first replaced by methyl groups with exhaustive methylation . After the conversion of the resulting quaternary halogen ammonium compounds into the hydroxide, the latter was thermally decomposed by pyrolysis. Depending on whether one, two or three methyl groups had been incorporated, this corresponded to primary, secondary or tertiary amines.
The second method uses the QAV property that the methyl group is bound to the amine nitrogen much more strongly than alkyl groups with more carbon atoms. During pyrolysis, no alcohol comparable to methanol is formed, but rather an alkene in addition to trimethylamine and water . This reaction is basically suitable for the preparation of alkenes . The reaction equation for the implementation is:
use
QAC with at least one long alkyl group have surface-active properties and are used as cationic surfactants in products such as fabric softeners , as invert soaps or as antistatic agents (e.g. in shampoos ). Due to their disinfecting effect, they are also counted among the biocides . They are used in public and industrial areas in hospitals , in food processing, in agriculture, in wood preservation and in industry (clean room applications). Quats are usually the main active ingredient in anti-algae agents ( algicides ) for swimming pools and pools. In addition, QACs are used as phase transfer catalysts in organic synthesis .
The QAV include, for example:
- Benzalkonium chloride
- Cetylalkonium chloride
- Cetyl pyridinium chloride
- Cetyltrimethylammonium bromide
- Denatonium benzoate
- Dimethyldioctadecylammonium chloride
- Tetrabutylammonium hydroxide (TBAH)
- Paraquat
- Polyquaternium-7
- Tetradecyl trimethyl ammonium oxalate
- 3-chloro-2-hydroxypropyl-N, N, N-trimethylammonium chloride
- Benzyl trimethyl ammonium chloride
QAV have recently also gained importance as ionic liquids .
Another area of application for QAV is water treatment , where they are used as strongly basic ion exchangers for the production of demineralized water . Type I of this anion exchanger is a trimethylbenzylammonium compound. This type I is thermally more stable than the exchangers of type II, in which at least one of the three methyl groups (-N + (CH 3 ) 3 ) of the quaternary amine is replaced by an ethyl group (-CH 2 -CH 2 -OH).
Biological importance
QAV accumulate in the cell membranes of living organisms and can thus impair the function of the cell membrane. Thanks to this effect, the cationic surfactants in particular can also be used as disinfectants . The microbicidal effect is only given if the alkyl group bonded to the N atom has a chain length of 8 to 18 C atoms. Many quaternary ammonium compounds are largely eliminated in sewage treatment plants through adsorption on the sewage sludge . The use of QAV-containing bathroom and toilet cleaners is not recommended. Hospitals and laundries were identified as the main sources. Because of their significantly better biodegradability , some QAV have been replaced by ester quats in recent years .
Natural occurrence
Choline occurs as a substance or chemically bound in numerous organisms. Acetylcholine , the acetic acid ester of choline, is an important neurotransmitter . Betaine is an oxidation product of choline and plays a role in transmethylation processes .
The fly agaric contains L - (+) - muscarine , a poisonous natural substance that is one of the quaternary ammonium compounds. The quaternary ammonium compound D - tubocurarine , which occurs in plants of the genus Chondrodendron, is part of the curare arrow poison.
The alkaloids sanguinarine , chelerythrine (e.g. found in celandine ) and berberine (e.g. in barberry ) have a strong red or yellow color due to their quaternary structure.
More recently there have been findings of QAV residues, in particular BAC (benzalkonium chloride) and DDAC (didecyldimethylammonium chloride) on fresh fruit and vegetables and in plant strengtheners.
nomenclature
In addition to the official IUPAC - nomenclature are to designate quaternary ammonium compounds, particularly with long alkyl chains, often trivial names of the alkyl groups used.
According to the IUPAC system, the names of these compounds are derived from the azanium ion.
See also
literature
- Chang Zhang, Fang Cui, Guang-ming Zeng, Min Jiang, Zhong-zhu Yang, Zhi-gang Yu, Meng-ying Zhu, Liu-qing Shen: Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. In: Science of the Total Environment 518-519, 2015, pp. 352-362, doi: 10.1016 / j.scitotenv.2015.03.007 .
Web links
- Quaternary ammonium compounds Information from the Austrian Federal Environment Agency
Individual evidence
- ↑ Entry on quaternary ammonium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- ↑ LF Fieser / M. Fieser; In: Textbook of Organic Chemistry ; Verlag Chemie, Weinheim / Bergstr .; 3rd edition, 1957, p. 248.
- ↑ LF Fieser / M. Fieser; In: Textbook of Organic Chemistry ; Verlag Chemie, Weinheim / Bergstr .; 3rd edition, 1957, p. 249.
- ↑ LF Fieser / M. Fieser; In: Textbook of Organic Chemistry ; Verlag Chemie, Weinheim / Bergstr .; 3rd edition, 1957, p. 260.
- ↑ G. Kühne and F. Martinola; In: Ion exchangers - their resistance to chemical and physical agents ; VGB Kraftwerkstechnik, 57, issue 3, March 1977, p. 176.
- ↑ Chang Zhang, Fang Cui, Guang-ming Zeng, Min Jiang, Zhong-zhu Yang, Zhi-gang Yu, Meng-ying Zhu, Liu-qing Shen: Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. In: Science of the Total Environment 518-519, 2015, pp. 352-362, doi: 10.1016 / j.scitotenv.2015.03.007 .
- ↑ a b Gans O. et al. (2005): Basics for risk assessment for quaternary ammonium compounds (PDF; 1.3 MB) . Federal Environment Agency, Vienna.
- ↑ Nadine Woodtli: “Kassensturz” test - the chemical club in the cleaning cupboard. In: srf.ch . November 26, 2019, accessed November 27, 2019 .
- ^ S. Mishra, VK Tyagi: Esterquats: the novel class of cationic fabric softeners. In: Journal of Oleo Science. Volume 56, Number 6, 2007, pp. 269-276, PMID 17898491 (review).
- ↑ Contamination of food by disinfectants .
- ^ GJ Leigh (Ed.): Principles of chemical nomenclature. A guide to IUPAC recommendations. The Royal Society of Chemistry, Cambridge 2011, p. 46.





