Ruthenium (VIII) oxide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ruthenium (VIII) oxide | |||||||||||||||
| other names |
Ruthenium tetroxide |
|||||||||||||||
| Molecular formula | RuO 4 | |||||||||||||||
| Brief description |
yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 165.07 g mol −1 | |||||||||||||||
| Physical state |
fixed at RT |
|||||||||||||||
| density |
3.29 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
25.4 ° C |
|||||||||||||||
| boiling point |
40 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−239 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ruthenium (VIII) oxide or ruthenium tetroxide is a chemical compound of ruthenium and the oxide with the highest oxidation level of the element. It is a yellow, volatile solid that is a strong oxidizing agent and reacts explosively with organic compounds.
history
Ruthenium (VIII) oxide was first isolated by Karl Ernst Claus in 1860 . He is also considered the discoverer of ruthenium.
Extraction and presentation
Ruthenium tetroxide can be obtained by oxidizing aqueous solutions of ruthenium (III) chloride or ruthenates with sodium periodate , sodium hypochlorite or sodium bromate . Due to its poor solubility in water, it escapes from the solution in gaseous form and can then be collected in suitable solvents.
properties
In ruthenium (VIII) oxide the ruthenium is in its maximum oxidation state +8. It is the highest known oxidation level for uncharged compounds and is only used for a few other compounds, such as B. Osmium tetroxide and xenon (VIII) oxide achieved. Only the IrO 4 + ion is an even higher oxidation state known (+9 for Iridium ).
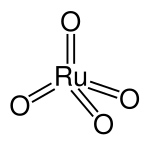
The ruthenium (VIII) oxide molecule has a tetrahedral structure and a Ru-O distance of 170.5 pm . It crystallizes in the form of yellow, rhombic needles and has a typical odor reminiscent of ozone.
Ruthenium tetroxide is thermally unstable and decomposes into ruthenium (IV) oxide and oxygen when heated . It is more unstable than the corresponding osmium analog. By potassium ruthenium tetroxide is reduced, it formed hexavalent ruthenate. The compound reacts explosively with ammonia , ethanol, oxidizable organic compounds, sulfur and hydrogen iodide .
use
Ruthenium (VIII) oxide is used as an oxidizing agent in organic chemistry. Examples are the Djerassi-Rylander oxidation , in which alkenes are split into carbonyl compounds , the oxidation of alcohols to aldehydes , ketones or carboxylic acids, or of alkynes to 1,2-diketones. Often it is generated in situ during the reaction.
In the separation of the platinum metals and the recovery of elemental ruthenium, ruthenium (VIII) oxide is an important intermediate product. By forming this compound, ruthenium can be separated from the other platinum metals.
In transmission electron microscopy , ruthenium (VIII) oxide is used as a contrast agent for polymers and biological samples. For this purpose, ruthenium (VIII) oxide is allowed to diffuse into the sample . If there are different compositions within the sample (e.g. polymer mixtures), this is usually associated with different diffusion speeds, which means that ruthenium (VIII) oxide accumulates to different degrees in the areas of different composition. However, since ruthenium scatters the electrons more strongly due to its comparatively high atomic number, contrast differences develop in the areas of different ruthenium concentrations.
safety instructions
Ruthenium (VIII) oxide is fire-promoting. In the event of contact with oxidizable substances (such as organic materials) there is a risk of explosion even at room temperature.
Individual evidence
- ↑ a b c d David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-86.
- ↑ a b c Entry on ruthenium (VIII) oxide in the GESTIS substance database of the IFA , accessed on April 13, 2010(JavaScript required) .
- ↑ a b c William P. Griffith: Ruthenium and Osmium Oxo Complexes as Organic Oxidants . In: Platinum Metals Review . tape 33 , no. 4 , 1989, pp. 181–185 ( platinummetalsreview.com [PDF; 357 kB ]).
- ↑ a b americanelements.com: CAS Number 20427-56-9 , accessed October 31, 2016
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 2: Subgroup elements, lanthanoids, actinides, transactinides. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049590-4 , p. 1975 (reading sample: Part C - Subgroup elements. Google book search ).
- ^ A b Hermann Renner et al .: Platinum Group Metals and Compounds. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2001, doi : 10.1002 / 14356007.a21_075 .
- ↑ Guanjun Wang, Mingfei Zhou, James T. Goettel, Gary G. Schrobilgen, Jing Su, Jun Li, Tobias Schlöder, Sebastian Riedel: Identification of an iridium-containing compound with a formal oxidation state of IX . In: Nature . tape 514 , August 21, 2014, p. 475-477 , doi : 10.1038 / nature13795 .
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1672–1673.
- ↑ a b Entry on ruthenium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on June 16, 2014.
