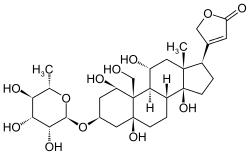
g-strophanthin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | g-strophanthin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 29 H 44 O 12 | |||||||||||||||||||||
| Brief description |
colorless, shiny crystals with a bitter taste |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Inhibition or activation of the Na + / K + -ATPase |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 584.65 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
190 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
10 g l −1 in water at 20 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
g-Strophanthin (from the Greek στροφή, "stanza", here in the sense of "turn, winding" related to the strophanthin-containing creeping plants, and ἄνϑος , "anthos" = flower) - also in common misspelling without the second h as strophantine - also ouabain , a cardenolide - glycoside , which as a cardiac glycoside has been used previously for the treatment of heart disease. The aglycon is g-strophanthidin (ouabagenin). Strophanthin acts on receptors on the enzyme Na + / K + -ATPase ( sodium-potassium pump ) and can be fatal in higher doses. In parts of Africa it was therefore used as an arrow poison.
Occurrence and nomenclature
g-Strophanthin is one of the strophanthines that occur in the seeds of various African creepers of the genus Strophanthus from the dog poison family . The letter g stands for the occurrence in the species Strophanthus gratus . The g-strophanthin can also be found in the Acokanthera plant ( Acokanthera oblongifolia , A. ouabaio and A. schimperi ), which can sometimes also be found in our pots. The term ouabain is derived from the African Ouabaio -tree (the scientific name Acokanthera ouabaio however, is a deprecated synonym; today is the kind Acokanthera oppositifolia ), whose seeds also contains ouabain. Ouabaio is the English spelling of the East African word Wabayo .
G-strophanthin, together with the k-strophanthin found in Strophanthus kombe, is one of the glycosides that have an effect on the heart (see cardiac glycosides ). The two substances are from out of the thimble ( Digitalis derived) digitalis to distinguish. The aglycon of k-strophanthin, which is also very poisonous k-strophantidine , is contained in the summer adonis ( Adonis aestivalis ), which is also native to Europe .
Strophanthin used to be one of the endogenous glycosides that function as hormones in mammals ; humans produce strophanthin in the adrenal cortex . There are now indications that it does not occur endogenously. During physical exertion, the synthesis of endogenous strophanthin should have increased, which should trigger the narrowing of blood vessels ( vasoconstriction ) and thus raise the arterial blood pressure. In mammals, with the exception of humans, strophanthin is stored in the spleen .
Effects
Higher concentrations of strophanthin, which can be achieved in the laboratory in a simple manner and clinically only by high doses of intravenously administered g-strophanthin, inhibit the sodium-potassium pump located in the cell membrane . The sodium-potassium pump (sodium-potassium-ATPase), which is particularly abundant in nerve and heart muscle cells , regulates the electrolyte concentration by pumping sodium ions out of the cell and potassium ions in. This inhibition is regarded as the classic effect of cardiac glycosides, which leads to an increase in the contraction force of the heart muscle cell via the increased cellular content of sodium and thus also calcium (via sodium-calcium exchanger ) (positive inotropic effect).
In low, physiological concentrations, such as those measured as hormones , after oral administration and also after slow intravenous injection in low doses, strophanthines, on the other hand, have a stimulating effect on the sodium-potassium pump , which leads to a reduction in the cellular sodium and calcium content.
In animal experiments it could be shown that g-strophanthin reduces the toxicity of digitalis due to the opposing cellular effects.
history
In western parts of Africa, an extract from the strophanthus seed was traditionally used as an arrow poison, among other things for elephant hunting.
After the botanist John Kirk discovered the powerful cardiac effects of accidentally ingested powdered Strophanthus kombé seeds during the Livingstone Expedition in 1859 and the Scottish pharmacologist and doctor Thomas Richard Fraser isolated the active ingredient as k-strophanthin in 1862, the g-strophanthin from Arnaud, a French chemist, isolated from Strophanthus gratus and the Ouabaio tree in 1888.
From 1865 alcoholic solutions of Strophanthus kombé seeds were used as a total extract, from 1885 quite often throughout Europe. However, uncertain concentration ratios and the accompanying substances with a laxative effect made the therapy difficult, even if it was used by many clinicians. From 1904 a standardized g-strophanthin solution was also available.
After private animal experiments in Heidelberg around 1900, the Baden doctor Albert Fraenkel tested intravenous k-strophanthin in heart patients in the Strasbourg University Clinic under the direction of Ludolf von Krehl . The success caused a sensation, and a year later the therapy was widespread. The preparation was commercially available as a combination from CF Boehringer & Soehne . In 1910, Rudolf Gottlieb and the pharmacologist Hans Horst Meyer wrote in the first edition of their pharmacology textbook that intravenous incorporation had "proven to be an important advance in therapy since the recommendation ... by Fraenkel and Schwartz". The 9th edition in 1936 reports something similar.
Areas of application were all heart diseases such as heart failure, rhythm anomalies, acute myocardial damage caused by z. B. Flu and diphtheria , digitalis intoxication, angina pectoris , heart attack and high blood pressure .
During the National Socialist dictatorship , strophanthin was occasionally used in concentration camps for the murder of prisoners. B. at Paul Schneider .
After the Second World War , oral digitalis preparations were also available, so that injections of strophanthin, which was difficult for doctor and patient, were used less often. After 1947 Boehringer Mannheim, in cooperation with the Stuttgart internist Berthold Kern, developed an oral preparation that consisted of 90% g- and 10% k-strophanthin, the strophoral, in tablet and drop form. In the course of time, a number of other preparations emerged, e.g. B. Strophinos drops, Purostrophan drops, Strodival capsules; the latter were the only ones on the market from 1984 until 2012.
Today's meaning
Strophanthin administered intravenously was recommended by textbooks for acute heart failure until 1992 because it is the fastest-acting cardiac glycoside. Today, the international guidelines generally advocate cardiac glycosides only in second place with regard to the treatment of chronic heart failure, but mostly for the use of digoxin .
ouabain improved similar to nitroglycerine the preload of the heart and the oxygen deficiency tolerance in patients with coronary insufficiency. However, the administration of strophantine is no longer relevant, since the pharmacokinetics are unpredictable for both oral and intravenous administration.
Although g-strophanthin has a moderately positive inotropic (strength-increasing) effect, the ascribed positive effects in the prevention and acute treatment of angina pectoris and myocardial infarction have only been proven in older studies from the 1950s to 1980s, most of which do not meet today's quality requirements corresponded to clinical studies .
Therefore, g-strophanthin does not play a role today in the guidelines for the treatment of acute coronary syndrome or chronic coronary artery disease .
Web links
- Y. Shah: Strophanthin - a special cardiac glycoside . (PDF) In: Journal for Complementary Medicine. 2, 2011, pp. 48-51.
literature
- Strophanthin: the true story of Hauke Fürstenwerth, Books on Demand, 2016, ISBN 9783739213521
Individual evidence
- ↑ a b Hermann Ammon (Ed.): Hunnius Pharmaceutical Dictionary . 8th edition, de Gruyter, Berlin 2004, ISBN 3-11-015792-6 .
- ↑ a b Entry on G-strophanthin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Entry for CAS no. 630-60-4 in the GESTIS substance database of the IFA , accessed on February 1, 2016 (JavaScript required)
- ↑ Entry on 3- (6-deoxy-α-L-mannopyranosyloxy) -1,5,11a, 14,19-pentahydroxycard-20 (22) -enolide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on 1 February 2016. Manufacturers or distributors can extend the harmonized classification and labeling .
- ↑ Serva: Safety data sheet according to 1907/2006 / EG, Article 31 of December 19, 2011 (PDF; 133 kB).
- ↑ a b Van de Werf F et al .: Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. EUR Heart J . 2008 Dec; 29 (23): 2909-45. PMID 19004841 .
- ↑ a b National Care Guideline of the German Medical Association, Chronic KHK, Version 1.8, April 2008. Online as PDF .
- ↑ a b Pfeilgift, in Deutsches Kolonial-Lexikon (1920), Volume III, p. 49
- ↑ W. Schoner, G. Scheiner-Bobis: Endogenous and exogenous cardiac glycosides and their mechanisms of action. In: American Journal of Cardiovascular Drugs . Volume 7, Number 3, 2007, pp. 173-189, PMID 17610345 . (Review).
- ↑ S. Baecher, M. Kroiss, M. Fassnacht, M. Vogeser: No endogenous ouabain is detectable in human plasma by ultra-sensitive UPLC-MS / MS. In: Clinica Chimica Acta . Volume 431, April 2014, pp. 87-92, doi : 10.1016 / j.cca.2014.01.038 . PMID 24508998 .
- ^ LK Lewis, TG Yandle, PJ Hilton, BP Jensen, EJ Begg, MG Nicholls: Endogenous ouabain is not ouabain. In: Hypertension. Volume 64, number 4, October 2014, pp. 680-683, doi : 10.1161 / HYPERTENSIONAHA.114.03919 . PMID 25001271 .
- ^ W. Schoner, G. Scheiner-Bobis: Role of endogenous cardiotonic steroids in sodium homeostasis . In: Nephrology Dialysis Transplantation . 2008, 23: pp. 2723-2729.
- ↑ Gao J, Wymore RS, Wang Y, et al. : Isoform-specific stimulation of cardiac Na / K pumps by nanomolar concentrations of glycosides . In: J. Gen. Physiol. . 119, No. 4, April 2002, pp. 297-312. PMID 11929882 . PMC 2238186 (free full text).
- ↑ Balzan S, D'Urso G, Nicolini G, Forini F, Pellegrino M, Montali U: Erythrocyte sodium pump stimulation by ouabain and an endogenous ouabain-like factor . In: Cell Biochem. Funct. . 25, No. 3, 2007, pp. 297-303. doi : 10.1002 / cbf.1387 . PMID 17191274 .
- ↑ Nesher M, Shpolansky U, Viola N, et al. : Ouabain attenuates cardiotoxicity induced by other cardiac steroids . In: Br. J. Pharmacol. . 160, No. 2, May 2010, pp. 346-54. doi : 10.1111 / j.1476-5381.2010.00701.x . PMID 20423344 .
- ↑ The poisonous secret of the Mane Rat .
- ↑ Albert Fraenkel and G. Schwartz: Treatises on digital therapy. I. About intravenous strophanthin injections in heart patients . In: Archives of Experimental Pathology and Pharmacology . 57, 1907, pp. 79-122. doi : 10.1007 / BF01841302 .
- ↑ Hans H. Meyer and R. Gottlieb: The experimental pharmacology as a basis of drug treatment, pp. 259-260. Urban & Schwarzenberg, Berlin and Vienna 1910.
- ↑ Hans H. Meyer and Ernst P. Pick: The experimental pharmacology as the basis of drug treatment. Ninth edition, p. 376. Urban & Schwarzenberg, Berlin and Vienna 1936.
- ↑ Ernst Edens : Digitalisfibel für den Doktor , p. 21, Julius Springer Verlag, Berlin 1941.
- ↑ Ernst Edens: Münch Med Wschr 1934, No. 37, pp. 1424-1427.
- ↑ Heinz Zimmermann: The clinical strophanthin theory of Edens in the light of new research results. Part I: Medical Clinic 1951, 46: 1028-1031 - Part II: Med Klin 1951, 46: 1049-1052.
- ↑ Fritz Meyer (University Clinic Cologne): Normal or subliminal strophanthine dosage. Klin Wschr 1936, 15: 1238-1241.
- ^ Walter Poller: Doctor's writer in Buchenwald , Offenbach a. M .: Verlag Das Segel, 1960; (Quoted from / after: Prediger in der Hölle, memorial booklet for the 25th anniversary of the death of Paul Schneider , Verlag Kirche und Mann, Gütersloh).
- ^ K. Luckhaupt-Koch: Characteristics of intensive care, in W. Dick (Ed.), With the participation of HP Schuster: Emergency and Intensive Medicine, De Gruyter Textbook, Berlin - NY, 1992, pp. 436-450, there p. 437.
- ↑ Lüllmann H & van Zwieten PA: The kinetic behavior of cardiac glycosides in vivo, measured by isotope techniques . In: Journal of Pharmacy and Pharmacology . 21: 1-8, 1969, p. 2.
- ↑ Guidelines for the therapy of chronic heart failure of the German Society for Cardiology (PDF, 391 kB).
- ↑ Belz GG, Matthews J, Sauer U, Stern H, Schneider B: Pharmacodynamic effects of ouabain following single sublingual and intravenous doses in normal subjects . In: Eur J Clin Pharmacol . 26, No. 3, 1984, pp. 287-92. PMID 6428911 .
- ↑ Sharma B, Majid PA, Meeran MK, Whitaker W, Taylor SH: Clinical, electrocardiographic, and haemodynamic effects of digitalis (ouabain) in angina pectoris . In: British Heart Journal . 34, No. 6, June 1972, pp. 631-7. PMID 4402698 . PMC 458511 (free full text).
- ↑ UC Hoppe, E. Erdmann. Guidelines for the therapy of chronic heart failure. Mitt Österr Ges Kardiol 1999; 2 (2): 9-16.
- ^ Fürstenwerth H: Ouabain - the insulin of the heart . In: Int. J. Clin. Pract. . 64, No. 12, November 2010, pp. 1591-4. doi : 10.1111 / j.1742-1241.2010.02395.x . PMID 20946265 .
- ↑ J. Wipplinger: Strophanthin: The Disappeared Heart Medication , Medizin-transparent.at, February 6, 2015.
