Triflumuron
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
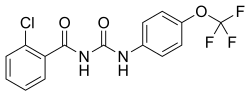
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Triflumuron | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 10 ClF 3 N 2 O 3 | ||||||||||||||||||
| Brief description |
colorless to white, crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 358.7 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.55 g cm −3 |
||||||||||||||||||
| Melting point |
194 ° C |
||||||||||||||||||
| boiling point |
decomposes at 360 ° C |
||||||||||||||||||
| Vapor pressure |
0.0002 mPa (25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Triflumuron is a synthetic insecticide from the active ingredient group of chitin biosynthesis inhibitors ( chitin inhibitors ) type 0. It was introduced in 1982 by Bayer CropScience .
Extraction and presentation
Triflumuron can be obtained by reacting 2-chlorobenzoic acid with ammonia , phosgene and 4-trifluoromethoxyaniline .
Mode of action
Triflumuron acts primarily as a food poison for biting and sucking pests. It disrupts the chitin biosynthesis of insects. As a result, larvae that come into contact with the active ingredient can no longer shed their skin, causing them to die. In addition, triflumuron was found to prevent larvae from hatching.
Areas of application
Triflumuron is used in fruit and vegetable cultivation against biting insects. It is also used in the cultivation of coffee, tea and cotton as well as in forestry.
In New Zealand and Australia it is used in sheep against lice ( Bovicola ovis ) under the name Bayer Zapp® Pour-on .
toxicology
Triflumuron is only very slightly acutely toxic for humans and mammals . It does not irritate the skin or the eyes and does not sensitize the skin . Furthermore, no genotoxic or carcinogenic properties of the substance could be demonstrated. The European Food Safety Authority EFSA set an ADI of 0.014 mg · kg −1 body weight.
Ecotoxicology
The effect of triflumuron on honey bees ( Apis mellifera ) was examined in a study. It was found that bees that were exposed to the active ingredient showed reduced flight activity. In addition, an increased mortality of the larvae could be detected. This effect lasts for a very long time, which means that triflumuron can be classified as highly toxic to bees.
Triflumuron is not very toxic to algae and fish , but highly toxic to aquatic invertebrates .
Analytics
For reliable detection and quantification of triflumuron, liquid and gas chromatographic methods can be used. A mass spectrometer can be used for identification after the chromatographic separation .
Admission
Triflumuron is approved as an insecticide in the European Union for the period from April 1, 2011 to March 31, 2021. In some countries, products with the active ingredient are approved, but not in Germany, Austria and Switzerland.
Trade names
Pesticides
- Bayer Alsystin® SC 480
- Bayer Starycide® SC
- Bayer Baycidal wettable powder
Veterinary drugs
- Bayer Zapp® Pour-on
- Bayer Zapp® ENCORE® Pour-on (combined preparation with Imidacloprid )
Individual evidence
- ↑ a b c Conclusion on the peer review of the pesticide risk assessment of the active substance triflumuron . In: EFSA Journal . tape 9 , no. 1 , January 2011, p. 1941 , doi : 10.2903 / j.efsa.2011.1941 .
- ↑ a b c d e f Entry on triflumuron in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on June 14, 2018.
- ↑ a b c d e data sheet triflumuron at Sigma-Aldrich , accessed on June 14, 2018 ( PDF ).
- ↑ a b c d Entry on triflumuron. In: Römpp Online . Georg Thieme Verlag, accessed on June 18, 2018.
- ↑ Thomas A. Unger: Pesticide synthesis handbook . Noyes Publications, 1996, ISBN 978-0-8155-1401-5 , pp. 259 ( limited preview in Google Book search).
- ↑ Simon R. Leather: Insect Reproduction . Ed .: Tylor & Francis Inc. CRC Press, Bosa Roca, United States 1995, ISBN 978-0-8493-6695-6 ( limited preview in Google book search).
- ↑ Zapp® Pour-on. In: bayeranimal.co.nz. Retrieved June 18, 2018 .
- ^ OG Amir, R. Peveling: Effect of triflumuron on brood development and colony survival of free-flying honeybee, Apis mellifera L. In: Journal of Applied Entomology . tape 128 , no. 4 , May 2004, p. 242-249 , doi : 10.1111 / j.1439-0418.2004.00782.x .
- ↑ Determination of pesticide residues in avocado and almond by liquid and gas chromatography tandem mass spectrometry. (PDF) In: eurl-pesticides.eu. Accessed June 18, 2018 .
- ↑ Implementing Regulation (EU) No. 540/2011 OF THE COMMISSION of 25 May 2011 for the implementation of Regulation (EC) No. 1107/2009 of the European Parliament and of the Council with regard to the list of approved active substances .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on triflumuron in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on June 18, 2018.

