User:Starblind and Light-emitting diode: Difference between pages
→Barnstars: +1 |
→Disadvantages of using LEDs: corrected a couple of mistakes |
||
| Line 1: | Line 1: | ||
{{redirect|LED}} |
|||
[[Image:Andrew_lenahan.jpg|frame|right|Andrew Lenahan in June 2007.]] |
|||
[[Image:User_starblind.jpg|frame|right|Interlocutor's Licence.]] |
|||
[[Image:RBG-LED.jpg|thumb|right|250px|Blue, green, and red LEDs; these can be combined to produce most perceptible colors, including white. |
|||
'''Andrew Lenahan''' is a writer and artist living in Virginia, in the United States. |
|||
<br><br> |
|||
Areas of expertise include old-time radio, British pop music, and mystery fiction. Andrew was elected as a Wikipedia administrator in 2005. |
|||
Infrared and ultraviolet (UVA) LEDs are also available.]] |
|||
[[Image:LED symbol.svg|right|thumb|250px|LED schematic symbol]] |
|||
==Further Information== |
|||
[[Image:Al Sheedakim and Panel.JPG|thumb|right|250px|[[LED panels]] allow for smaller sets of interchangeable LEDs to be one large display.]] |
|||
* Website and weblog: http://www.starblind.com |
|||
* Photography and Paintings: http://starblind.deviantart.com |
|||
* MySpace page: http://www.myspace.com/andrew_lenahan |
|||
A '''light-emitting diode''' ('''LED''') ({{pronEng|ˌɛliːˈdiː}}),<ref>{{cite web |
|||
==Wikipedia Activity== |
|||
| url = http://en.wiktionary.org/wiki/LED |
|||
| title = LED |
|||
| accessdate = 2008-01-04 |
|||
}}</ref> is a [[semiconductor]] [[diode]] that emits light when an [[electric current]] is applied in the forward direction of the device, as in the simple [[LED circuit]]. The effect is a form of [[electroluminescence]] where [[coherence (physics)|incoherent]] and narrow-[[spectrum]] light is emitted from the [[p-n junction]]. |
|||
LEDs are widely used as indicator lights on electronic devices and increasingly in higher power applications such as flashlights and area [[lighting]]. |
|||
===Articles originated or raised from substub level:=== |
|||
An LED is usually a small area (less than 1 mm<sup>2</sup>) light source, often with optics added to the chip to shape its radiation pattern and assist in reflection.<ref>{{cite paper |
|||
[[Argei]] | [[Aunt Jenny]] | [[Axehandle hound]] | [[Beautiful Joe]] | [[Bionic Bunny]] | [[Bobblehead]] | [[Brannock Device]] | [[The Budget]] | [[California dissanthelium]] | [[The Campaign for North Africa]] | [[Charley Says]] | [[Chimera Beast]] | [[Cookie Puss]] | [[Boston Custer]] | [[Delightes for Ladies]] | [[The Eagles (UK band)]] | [[Edward Mabry]] | [[The Frim Fram Sauce]] | [[Noel Harrison]] | [[Heinz pickle pin]] | [[Human-animal communication]] | [[If You Go Away]] | [[Keraunography]] | [[Little Dot]] | [[Reg Manning]] | [[Maryland Scroll]] | [[Maxi single]] | [[Mick Inkpen]] | [[Mycosis fungoides]] | [[Nippy]] | [[Nude on the Moon]] | [[Dick Pointer]] | [[Queer Duck]] | [[Rabbit rabbit]] | [[Charley Ross]] | [[Sacred Contagion]] | [[Saengerfest]] | [[Shadrach]] | [[Shrine Records]] | [[Snail Maze]] | [[Super Star Wars]] | [[Super Star Wars: The Empire Strikes Back]] | [[Super Star Wars: Return of the Jedi]] | [[Supertaster]] | [[TerraTopia]] | [[Tingha and Tucker]] | [[Toile]] | [[Where's Huddles?]] | [[Wibbly Pig]] | [[Chuck Williams]] | [[Women Wise]] | [[Doris Wishman]] | [[Hugh H. Young]] | [[Zoe Motors]] |
|||
|url=http://www.opticsexpress.org/viewmedia.cfm?id=149957&seq=0 |
|||
|title=Modeling the radiation pattern of LEDs |
|||
|work=... |
|||
|author=... |
|||
|publisher=Optics Express |
|||
|date=2008 |
|||
|accessdate=2008-01-25}} |
|||
</ref><ref>{{cite paper |
|||
|url=http://planck.reduaz.mx/~imoreno/Publicaciones/IODC2006.pdf |
|||
|title=LED Intensity Distribution |
|||
|work=International Optical Design Conference |
|||
|author=I. Moreno |
|||
|publisher=International Optical Design, Technical Digest |
|||
|date=2006 |accessdate=2007-08-13}} |
|||
</ref> |
|||
The [[color]] of the emitted light depends on the composition and condition of the semiconducting material used, and can be [[infrared]], [[visible spectrum|visible]], or [[ultraviolet]]. Besides lighting, interesting applications include using [[UV]]-LEDs for sterilization of water and disinfection of devices,<ref name=water sterilization">[http://www.springerlink.com/content/668j066286443722/ Development of a new water sterilization device with a 365 nm UV-LED, Medical and Biological Engineering and Computing, Volume 45, Number 12 / December, 2007]</ref> and as a [[grow light]] to enhance [[photosynthesis]] in plants.<ref>{{cite paper |
|||
|title=Light-emitting diodes as a light source for photosynthesis research |
|||
|author=Tennessen, D.J. and Singsaas, E.L. and Sharkey, T.D. |
|||
|journal=Photosynthesis Research |
|||
|publisher=Springer |
|||
|date=1994 |
|||
|pages=85-92 |
|||
|accessdate=2008-07-24}} |
|||
</ref> |
|||
==History== |
|||
===Articles significantly added upon:=== |
|||
=== Discovery and development=== |
|||
[[Carter's Ink]] | [[Jigglypuff]] | [[Mannequin]] | [[Mary Pearcey]] | [[N-Gage]] | [[Swedish Chef]] | [[Bruce Weber]] |
|||
The first known report of a light-emitting solid-state diode was made in 1907 by the British experimenter [[H. J. Round]] of [[Marconi Labs]].<ref>{{cite journal |
|||
|author=H. J. Round |
|||
|year=1907 |
|||
|title=A Note on Carborundum |
|||
|journal=Electrical World |
|||
|volume=19 |
|||
|pages=309}} |
|||
</ref> Russian [[Oleg Vladimirovich Losev]] independently created the first LED in the mid 1920s; his research, though distributed in Russian, German and British scientific journals, was ignored,<ref name="Zheludev_100yearhistory">{{cite journal |
|||
|author=Zheludev, N. |
|||
|year=2007 |
|||
|title=The life and times of the LED — a 100-year history |
|||
|journal=Nature Photonics |
|||
|volume=1 |
|||
|issue=4 |
|||
|pages=189–192 |
|||
|url=http://www.nanophotonics.org.uk/niz/publications/zheludev-2007-ltl.pdf |
|||
|format=PDF |
|||
|doi = 10.1038/nphoton.2007.34 <!--Retrieved from CrossRef by DOI bot-->}} |
|||
</ref><ref>{{cite web |
|||
|url=http://www.jmargolin.com/history/trans.htm |
|||
|author=Margolin J |
|||
|title=''The Road to the Transistor''}} |
|||
</ref> and no practical use was made of the discovery for several decades. [[Rubin Braunstein]] of the [[Radio Corporation of America]] reported on infrared emission from [[gallium arsenide|gallium arsenide (GaAs)]] and other semiconductor alloys in 1955.<ref>{{cite paper |
|||
|author=Braunstein, Rubin |
|||
|year=1955. |
|||
|title="Radiative Transitions in Semiconductors," |
|||
|journal=Physical Review |
|||
|volume=99 |
|||
|pages=1892-3. |
|||
|url=http://prola.aps.org/abstract/PR/v99/i6/p1892_1 |
|||
|doi=10.1103/PhysRev.99.1892}} |
|||
</ref> Braunstein observed infrared emission generated by simple diode structures using [[Gallium antimonide|GaSb]], [[GaAs]], [[Indium phosphide|InP]], and Ge-Si alloys at room temperature and at 77 K. In 1961, experimenters Bob Biard and Gary Pittman working at [[Texas Instruments]],<ref>{{cite web |
|||
|url=http://invention.smithsonian.org/centerpieces/quartz/inventors/biard.html |
|||
|title=The first LEDs were infrared (invisible) |
|||
|work=The Quartz Watch |publisher=The Lemelson Center |
|||
|accessdate=2007-08-13}} |
|||
</ref> found that gallium arsenide gave off infrared radiation when electric current was applied. Biard and Pittman were able to establish the priority of their work and received the patent for the infrared light-emitting [[diode]]. |
|||
The first practical visible-spectrum (red) LED was developed in 1962 by [[Nick Holonyak|Nick Holonyak Jr.]], while working at [[General Electric Company]]. He later moved to the [[University of Illinois at Urbana-Champaign]].<ref>{{cite web |
|||
===My wiki-related articles=== |
|||
|url=http://web.mit.edu/invent/a-winners/a-holonyak.html |
|||
|title=Nick Holonyak, Jr. 2004 Lemelson-MIT Prize Winner |
|||
|publisher=Lemenson-MIT Program |accessdate=2007-08-13}} |
|||
</ref> Holonyak is seen as the "father of the light-emitting diode".<ref name="chisuntimes">{{cite news |
|||
| title = U. of I.'s Holonyak out to take some of Edison's luster |
|||
| author = Wolinsky, Howard |
|||
| date = [[February 5]] [[2005]] |
|||
| url = http://findarticles.com/p/articles/mi_qn4155/is_20050202/ai_n9504926 |
|||
| publisher = ''Chicago Sun-Times'' |
|||
| accessdate = 2007-07-29 }}</ref> |
|||
M. George Craford, a former graduate student of Holonyak's, invented the first yellow LED and 10x brighter red and red-orange LEDs in 1972.<ref>{{cite web |
|||
|url=http://www.technology.gov/Medal/2002/bios/Holonyak_Craford_Dupuis.pdf |
|||
|title=Brief Biography – Holonyak, Craford, Dupuis |
|||
|publisher=Technology Administration |
|||
|format=PDF |
|||
|accessdate=2007-05-30}} |
|||
</ref> |
|||
[[Shuji Nakamura]] of [[Nichia Corporation]] of Japan demonstrated the first high-brightness blue LED based on [[Indium gallium nitride|InGaN]] borrowing on critical developments in [[Gallium nitride|GaN]] nucleation on sapphire substrates and the demonstration of p-type doping of GaN which were developed by I. Akasaki and H. Amano in [[Nagoya]]. In 1995, [[Alberto Barbieri]] at the [[Cardiff University]] Laboratory (GB) investigated the efficiency and reliability of high-brightness LEDs demonstrating very high result by using a transparent contact made of [[indium tin oxide]] (ITO) on (AlGaInP/GaAs) LED. The existence of blue LEDs and high efficiency LEDs quickly led to the development of the first white LED, which employed a Y<sub>3</sub>Al<sub>5</sub>O<sub>12</sub>:Ce, or "[[YAG]]", phosphor coating to mix yellow (down-converted) light with blue to produce light that appears white. Nakamura was awarded the 2006 [[Millennium Technology Prize]] for his invention.<ref>{{cite web |
|||
*[[User:Starblind/Inclusion|Inclusion]] - What, in my opinion, makes a topic worthy of an article |
|||
|url=http://www.ia.ucsb.edu/pa/display.aspx?pkey=1475 |
|||
*[[User:Starblind/DeletionWars|Deletion Wars]] Who won the war between the Deletionists and Inclusionists? |
|||
|title=2006 Millennium technolgy prize awarded to UCSB's Shuji Nakamura |
|||
*[[User:Starblind/Interesting|Interesting]] - Some interesting and unusual articles. |
|||
|accessdate=2007-05-30}}</ref> |
|||
The development of LED technology has caused their efficiency and light output to increase [[exponentially]], with a doubling occurring about every 36 months since the 1960s, in a similar way to [[Moore's law]]. The advances are generally attributed to the parallel development of other semiconductor technologies and advances in optics and material science. This trend is normally called [[Haitz's Law]] after Dr. Roland Haitz. |
|||
===Other important stuff=== |
|||
*[http://mail.wikipedia.org/pipermail/foundation-l/2006-September/010610.html Wikimedia policy change] on vanity and spam. Refreshing and most welcome! |
|||
=== Practical use === |
|||
==Quotes of considerable interest== |
|||
[[Image:Charger Squad Car Rear Quarter Shot.jpg|thumb|right|Some police vehicle [[lightbar]]s incorporate LED technology.]] |
|||
The first commercial LEDs were commonly used as replacements for [[incandescence|incandescent]] indicators, and in [[seven-segment display]]s, first in expensive equipment such as laboratory and electronics test equipment, then later in such appliances as TVs, radios, telephones, calculators, and even watches (see list of [[Led#Indicators_and_signs|signal applications]]). These red LEDs were bright enough only for use as indicators, as the light output was not enough to illuminate an area. Later, other colors became widely available and also appeared in appliances and equipment. As the LED materials technology became more advanced, the light output was increased, while maintaining the efficiency and the reliability to an acceptable level, causing LEDs to become bright enough to be used for illumination, in [[Led#Lighting|various applications]] such as lamps and other lighting fixtures. |
|||
Most LEDs were made in the very common 5 mm T1³⁄₄ and 3 mm T1 packages, but with higher power, it has become increasingly necessary to shed excess heat in order to maintain reliability, so more complex packages adapted for efficient heat dissipation are becoming common. Packages for state-of-the-art [[Led#High_power_LEDs|high power LEDs]] bear little resemblance to early LEDs. |
|||
"What is history but a fable agreed upon?" - Napoleon Bonaparte |
|||
== LED technology == |
|||
"There's never been a golden age, nostalgia's for the lame. The best is always yet to come, it's that kind of game." - Stephen Duffy |
|||
=== Physical principles === |
|||
[[Image:PnJunction-LED-E.PNG|thumb|300px|right|The inner workings of an LED]] |
|||
[[Image:Rectifier vi curve.GIF|thumb|300px|right|I-V diagram for a [[diode]] an LED will begin to emit light when the on-[[voltage]] is exceeded. Typical on voltages are 2-3 [[Volt]]]] |
|||
Like a normal [[diode]], the LED consists of a chip of semiconducting material impregnated, or ''[[Doping (semiconductor)|doped]]'', with impurities to create a ''[[p-n junction]]''. As in other diodes, current flows easily from the p-side, or [[anode]], to the n-side, or [[cathode]], but not in the reverse direction. Charge-carriers—[[electron]]s and [[electron hole|holes]]—flow into the junction from [[electrode]]s with different [[voltage]]s. When an electron meets a hole, it falls into a lower [[energy level]], and releases [[energy]] in the form of a [[photon]]. |
|||
The [[wavelength]] of the light emitted, and therefore its color, depends on the [[band gap]] energy of the materials forming the ''p-n junction''. In [[silicon]] or [[germanium]] diodes, the electrons and holes recombine by a ''non-radiative transition'' which produces no optical emission, because these are [[indirect band gap]] materials. The materials used for the LED have a [[direct band gap]] with energies corresponding to near-infrared, visible or near-ultraviolet light. |
|||
"We know that under the revealed image there is another one which is more faithful to reality, and under this one there is yet another, and again another under this last one, down to the true image of what absolute, mysterious reality that nobody will ever see. Or perhaps, not until the decomposition of every image, every reality." - Michelangelo Antonioni |
|||
LED development began with infrared and red devices made with [[gallium arsenide]]. Advances in [[materials science]] have made possible the production of devices with ever-shorter [[wavelength]]s, producing light in a variety of colors. |
|||
==Barnstars== |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
|rowspan="2" valign="middle" | [[Image:Barnstar_of_Diligence.png|100px]] |
|||
|rowspan="2" | |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''The Barnstar of Diligence''' |
|||
|- |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | Awarded for diligent research on [[Axehandle hound]], as well as an excellent rewrite. '''[[User:Coredesat|Core]][[User:Coredesat/Esperanza|<font color="green">des</font>]][[User:Coredesat|at]]''' <small>[[User talk:Coredesat|talk. ^_^]]</small> 00:50, 30 July 2006 (UTC) ________________________________________________________________ |
|||
|} |
|||
LEDs are usually built on an n-type substrate, with an electrode attached to the p-type layer deposited on its surface. P-type substrates, while less common, occur as well. Many commercial LEDs, especially GaN/InGaN, also use [[sapphire]] substrate. |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
|rowspan="2" valign="middle" | [[Image:Barnstar of Humour3.png|100px]] |
|||
|rowspan="2" | |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''The Barnstar of Good Humor''' |
|||
|- |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | I award you the Barnstar of Good Humor, for your deletion review of [[Aice5]]. But what the hell does low-grit sandpaper on the arse of a wooly mammoth with a bad case of piles sound like?? :-) [[Wikipedia:Requests for adminship/Aecis|<font color="blue">A</font>]][[User:Aecis|<font color="green">ecis</font>]] <sup>[[Special:Contributions/Aecis|Dancing]] to electro-pop [[User talk:Aecis|like a robot]] from 1984.</sup> 11:18, 27 October 2006 (UTC) ________________________________________________________________ |
|||
|} |
|||
===Light extraction=== |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
The [[refractive index]] of most LED semiconductor materials is quite high, so in almost all cases the light from the LED is coupled into a much lower-index medium. The large index difference makes the [[reflection]] quite substantial (per the [[Fresnel equations|Fresnel coefficients]]). The produced light gets partially reflected back into the semiconductor, where it may be absorbed and turned into additional heat; this is usually one of the dominant causes of LED inefficiency. Often more than half of the emitted light is reflected back at the LED-package and package-air interfaces. |
|||
|rowspan="2" valign="middle" | [[Image:Barnstar of Diligence.png|100px]] |
|||
|rowspan="2" | |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''The Barnstar of Diligence''' |
|||
|- |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | Starblind, this barnstar is for you; you've kept going even when things have started to get tough, and you're an excellent admin, keep the great work up! [[User:SunStar Net|SunStar Net]] 20:00, 8 November 2006 (UTC) ________________________________________________________________ |
|||
|} |
|||
The reflection is most commonly reduced by using a dome-shaped (half-sphere) package with the diode in the center so that the outgoing light rays strike the surface [[perpendicularly]], at which angle the reflection is minimized. Substrates that are transparent to the emitted wavelength, and backed by a reflective layer, increase the LED efficiency. The refractive index of the package material should also match the index of the semiconductor, to minimize back-reflection. An [[anti-reflection coating]] may be added as well. |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
|rowspan="2" valign="middle" | [[Image:Original Barnstar.png|100px]] |
|||
|rowspan="2" | |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''Barnstar''' |
|||
|- |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | This Barnstar is awarded to you for your great work on the [[Bionic Bunny]] article. --[[User:ike9898|ike9898]] 04:44, August 6, 2005 (UTC) ________________________________________________________________ |
|||
|} |
|||
The package may be colored, but this is only for cosmetic reasons or to improve the contrast ratio; the color of the packaging does not substantially affect the color of the light emitted. |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
|rowspan="2" valign="middle" | [[Image:Working_Man's_Barnstar.png|100px]] |
|||
|rowspan="2" | |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''The Working Man's Barnstar''' |
|||
|- |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | For all your work on [[Wikipedia:Deletion review]], [[Wikipedia:Articles for deletion]] and [[Wikipedia:Miscellany for deletion]], and for being one of my favourite admins here! <font color="Red">[[User:SunStar Net|'''SunStar Net''']]</font> <sup><font color="Blue">[[User talk:SunStar Net|''talk'']]</font></sup> 18:11, 2 April 2007 (UTC) ________________________________________________________________ |
|||
|} |
|||
Other strategies for reducing the impact of the interface reflections include designing the LED to reabsorb and reemit the reflected light (called ''photon recycling'') and manipulating the microscopic structure of the surface to reduce the reflectance, by introducing random roughness, creating programmed ''moth eye'' surface patterns. Recently [[photonic crystal]] have also been used to minimize back-reflections.<ref>{{cite paper |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
|author = Cho, H.K., Jang, J., Choi, J.H., Choi, J., Kim, J., Lee, J.S., Lee, B., Choe, Y.H., Lee, K.D., Kim, S.H. and others |
|||
|rowspan="2" valign="middle" | [[Image:Barnstar rescue 04.png|100px]] |
|||
|title = Light extraction enhancement from nano-imprinted photonic crystal GaN-based blue light-emitting diodes, |
|||
|rowspan="2" | |
|||
|journal = Optics Express, |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''Rescue Barnstar''' |
|||
|year = 2006, |
|||
|- |
|||
|volume = 14, |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | I gladly present Starblind with this rescue award, for saving my Axehandle Hound page. !paradigm! 00:59, 13 August 2006 (UTC) ________________________________________________________________ |
|||
|pages = 8654--8660 |
|||
|} |
|||
|number = 19 |
|||
|publisher = OSA |
|||
}}</ref> |
|||
In December 2007, scientists at [[Glasgow University]] claimed to have found a way to make LEDs more energy efficient, imprinting billions of holes into LEDs using a process known as [[nanoimprint lithography]].<ref Name="BBC_Rahman">{{cite web |
|||
| title = New efficient bulb sees the light |
|||
| work = A new type of super-efficient household light bulb is being developed which could spell the end of regular bulbs. |
|||
| publisher = BBC News |
|||
| date = Friday, [[28 December]] [[2007]] |
|||
| url = http://news.bbc.co.uk/2/hi/uk_news/scotland/glasgow_and_west/7162606.stm |
|||
| format = Web |
|||
| doi = |
|||
| accessdate = 2008-01-01}}</ref> |
|||
===Colors and Materials=== |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
Conventional LEDs are made from a variety of inorganic [[semiconductor materials]], the following table shows the available colors with wavelength range, voltage drop and material: |
|||
|rowspan="2" valign="middle" | [[Image:Pokebarnstar-v2.png|100px]] |
|||
|rowspan="2" | |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''PokeBarnstar''' |
|||
|- |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | And this one for your fantastic work in [[WP:PAC]]. [[User:Almafeta|Almafeta]] 10:36, 13 August 2005 (UTC) ________________________________________________________________ |
|||
|} |
|||
{| class=wikitable |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
!Color |
|||
|rowspan="2" valign="middle" | [[Image:Original Barnstar.png|100px]] |
|||
![[Wavelength]] (nm) |
|||
|rowspan="2" | |
|||
![[Voltage]] (V) |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''Barnstar''' |
|||
!Semi-conductor Material |
|||
|- |
|- |
||
|[[Infrared]] ||[[Wavelength|λ]] > 760 ||[[Delta (letter)|Δ]]V < 1,9 || [[Gallium arsenide]] (GaAs)<br /> [[Aluminium gallium arsenide]] (AlGaAs) |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | I award this [[Wikipedia:Barnstars on Wikipedia|Barnstar]] to Starblind for his consistently useful comments in [[WP:AFD|Articles for Deletion]] discussions. [[User:Dark Shikari|<span style="background-color:#DDDDFF; font-weight:bold"><FONT COLOR="#0000FF">Da</FONT><FONT COLOR="#0000CC">rk</FONT> <FONT COLOR="#000099">Sh</FONT><FONT COLOR="#000066">ik</FONT><FONT COLOR="#000033">ar</FONT><FONT COLOR="#000000">i</FONT>]] <font color="#000088"><sup>[[User_talk:Dark_Shikari|''talk'']] 19:32, 17 October 2006 (UTC)</font></font> ________________________________________________________________ |
|||
|} |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
|rowspan="2" valign="middle" | [[Image:Ok_openclipart.png|100px]] |
|||
|rowspan="2" | |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''This user is <u>OK!</u>''' |
|||
|- |
|- |
||
|[[Red]] ||610 < λ < 760 ||1,63 < Δ[[Voltage|V]] < 2,03 || [[Aluminium gallium arsenide]] (AlGaAs)<br />[[Gallium arsenide phosphide]] (GaAsP)<br \>[[Aluminium gallium indium phosphide]] (AlGaInP) |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | You are hereby awarded the [[User:Infrangible|Infrangible]] seal of approval for outstanding contributions and overall coolness. <font color="#000066">'''[[User:Infrangible|~ Infrangible]]'''</font> 17:28, 15 July 2007 (UTC) ________________________________________________________________ |
|||
|} |
|||
{| style="border: 1px solid gray; background-color: #fdffe7;" |
|||
|rowspan="2" valign="middle" | [[Image:SpecialBarnstar.png|100px]] |
|||
|rowspan="2" | |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''The Special Barnstar''' |
|||
|- |
|- |
||
|[[Orange (colour)|Orange]] ||590 < λ < 610 ||2,03 < ΔV < 2,10 || [[Gallium arsenide phosphide]] (GaAsP)<br \>[[Aluminium gallium indium phosphide]] (AlGaInP) |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | In celebration of your important, impressive and invaluable contributions to Wikipedia. [[User:Ecoleetage|Ecoleetage]] ([[User talk:Ecoleetage|talk]]) 23:22, 20 June 2008 (UTC) |
|||
|- |
|||
|[[Yellow]] ||570 < λ < 590 ||2,10 < ΔV < 2,18 || [[Gallium arsenide phosphide]] (GaAsP)<br \>[[Aluminium gallium indium phosphide]] (AlGaInP) |
|||
|- |
|||
|[[Green]] ||500 < λ < 570 ||2,18 < ΔV < 4,0 || [[Indium gallium nitride]] (InGaN) / [[Gallium(III) nitride]] (GaN)<br />[[Gallium(III) phosphide]] (GaP)<br \>[[Aluminium gallium indium phosphide]] (AlGaInP)<br \>[[Aluminium gallium phosphide]] (AlGaP) |
|||
|- |
|||
|[[Blue]] ||450 < λ < 500 ||2,48 < ΔV < 3,7 || [[Zinc selenide]] (ZnSe)<br />[[Indium gallium nitride]] (InGaN)<br />[[Silicon carbide]] (SiC) as substrate<br \>[[Silicon]] (Si) as substrate — (under development) |
|||
|- |
|||
|[[Violet (color)|Violet]] ||400 < λ < 450 ||2,76 < ΔV < 4,0 || [[Indium gallium nitride]] (InGaN) |
|||
|- |
|||
|[[Ultraviolet]] ||λ < 400 ||3,1 < ΔV < 4,4 || [[diamond]] (C)<br \>[[Aluminium nitride]] (AlN)<br \> [[Aluminium gallium nitride]] (AlGaN)<br \> [[Aluminium gallium indium nitride]] (AlGaInN) — (down to 210 nm<ref>{{cite news |url=http://physicsworld.com/cws/article/news/24926 |title=LEDs move into the ultraviolet |date=[[May 17]] [[2006]] |publisher=physicsworld.com |accessdate=2007-08-13}}</ref>) |
|||
|- |
|||
|[[White]] || Broad spectrum ||ΔV = 3,5 || Blue/UV diode with phosphor |
|||
|} |
|} |
||
=== Ultraviolet and blue LEDs === |
|||
{| style="border: 1px solid {{{border|gray}}}; background-color: {{{color|#fdffe7}}};" |
|||
|rowspan="2" valign="middle" | [[Image:Barnstar rescue 04.png|90px]] |
|||
[[Image:Blue LED and Reflection.JPG|thumb|right|230px|[[Blue]] LEDs.]] |
|||
|rowspan="2" | |
|||
|style="font-size: x-large; padding: 0; vertical-align: middle; height: 1.1em;" | '''The Rescue from Deletion Barnstar''' |
|||
Blue LEDs are based on the wide [[band gap]] semiconductors GaN ([[gallium nitride]]) and [[InGaN]] (indium gallium nitride). They can be added to existing red and green LEDs to produce the impression of [[white]] light, though white LEDs today rarely use this principle. |
|||
The first blue LEDs were made in 1971 by Jacques Pankove (inventor of the gallium nitride LED) at [[RCA|RCA Laboratories]].<ref>{{cite web |
|||
| title = Alumni society honors four leaders in engineering and technology |
|||
| work = Berkeley Engineering News |
|||
| publisher = |
|||
| date = [[2000-09-04]] |
|||
| url = http://www.coe.berkeley.edu/EPA/EngNews/00F/EN2F/deaa.html |
|||
| format = |
|||
| doi = |
|||
| accessdate = 2007-01-23 }}</ref> However, these devices had too little light output to be of much practical use. In the late 1980s, key breakthroughs in GaN [[epitaxial]] growth and [[p-type]] doping by [[Isamu Akasaki]] and Hiroshi Amano (Nagoya, Japan)<ref>{{cite web |
|||
| title = GaN-based blue light emitting device development by Akasaki and Amano |
|||
| work = Takeda Award 2002 Achievement Facts Sheet |
|||
| publisher = The Takeda Foundation |
|||
| date = [[2002-04-05]] |
|||
| url = http://www.takeda-foundation.jp/en/award/takeda/2002/fact/pdf/fact01.pdf |
|||
| format = pdf |
|||
| doi = |
|||
| accessdate = 2007-11-28 }}</ref> ushered in the modern era of GaN-based optoelectronic devices. Building upon this foundation, in 1993 high brightness blue LEDs were demonstrated through the work of [[Shuji Nakamura]] at [[Nichia Corporation]].<ref>{{cite web |
|||
| title = United States Patent No. 5,578,839 (Nakamura et al.) |
|||
| work = |
|||
| publisher = [[United States Patent and Trademark Office]] |
|||
| date = filed [[1993-11-17]] |
|||
| url = http://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN%2F5578839 |
|||
| format = |
|||
| doi = |
|||
| accessdate = 2007-01-23 }}</ref> |
|||
By the late 1990s, blue LEDs had become widely available. They have an active region consisting of one or more InGaN [[quantum well]]s sandwiched between thicker layers of GaN, called cladding layers. By varying the relative InN-GaN fraction in the InGaN quantum wells, the light emission can be varied from violet to amber. AlGaN [[aluminium gallium nitride]] of varying AlN fraction can be used to manufacture the cladding and quantum well layers for ultraviolet LEDs, but these devices have not yet reached the level of efficiency and technological maturity of the InGaN-GaN blue/green devices. If the active quantum well layers are GaN, as opposed to alloyed InGaN or AlGaN, the device will emit near-ultraviolet light with wavelengths around 350–370 nm. Green LEDs manufactured from the InGaN-GaN system are far more efficient and brighter than green LEDs produced with non-nitride material systems. |
|||
With nitrides containing aluminium, most often [[Aluminium gallium nitride|AlGaN]] and [[aluminium gallium indium nitride|AlGaInN]], even shorter wavelengths are achievable. Ultraviolet LEDs in a range of wavelengths are becoming available on the market. Near-UV emitters at wavelengths around 375–395 nm are already cheap and often encountered, for example, as [[black light]] lamp replacements for inspection of anti-[[counterfeiting]] UV watermarks in some documents and paper currencies. Shorter wavelength diodes, while substantially more expensive, are commercially available for wavelengths down to 247 nm.<ref>[http://www.s-et.com/products.htm Sensor Electronic Technology, Inc.: Nitride Products Manufacturer]</ref> As the photosensitivity of microorganisms approximately matches the absorption spectrum of [[DNA]], with a peak at about 260 nm, UV LEDs emitting at 250–270 nm are to be expected in prospective disinfection and sterilization devices. Recent research has shown that commercially available UVA LEDs (365 nm) are already effective disinfection and sterilization devices.<ref name=water sterilization"/> |
|||
Wavelengths down to 210 nm were obtained in laboratories using [[aluminium nitride]]. |
|||
While not an LED as such, an ordinary NPN bipolar transistor will emit violet light if its emitter-base junction is subjected to non-destructive reverse breakdown. This is easy to demonstrate by filing the top off a metal-can transistor (BC107, 2N2222 or similar) and biasing it well above emitter-base breakdown (≥ 20 V) via a current-limiting resistor. |
|||
=== White light LEDs === |
|||
There are two ways of producing high intensity [[white]]-light using LEDs. One is to use individual LEDs that emit three [[primary color]]s<ref>{{cite news |
|||
| title = Jan Henrik Wold and Arne Valberg |
|||
| publish = Vol. 26, pp. S222 |
|||
| date = 2001}}</ref> – red, green, and blue, and then mix all the colors to produce [[white]] light. The other is to use a phosphor material to convert monochromatic light from a blue or UV LED to broad-spectrum white light, much in the same way a fluorescent light bulb works. |
|||
==== RGB Systems ==== |
|||
[[Image:Red-YellowGreen-Blue LED spectra.png|thumb|right|350px|Combined spectral curves for blue, yellow-green, and high brightness red solid-state semiconductor LEDs. [[Full width at half maximum|FWHM]] spectral bandwidth is approximately 24–27 nm for all three colors.]] |
|||
[[White_light#Light|White light]] can be produced by mixing differently colored light, the most common method is to use [[rgb|red, green and blue]] (RGB). Hence the method is called multi-colored white LEDs (sometimes referred to as RGB LEDs). Because its mechanism is involved with sophisticated electro-optical design to control the blending and [[diffusion]] of different colors, this approach has rarely been used to mass produce white LEDs in the industry. Nevertheless this method is particularly interesting to many researchers and scientists because of the flexibility of mixing different colors <ref>{{cite paper |url=http://www.opticsexpress.org/viewmedia.cfm?uri=oe-15-6-3607&seq=0 |title=Color distribution from multicolor LED arrays |work=... |author=... |publisher=Optics Express |date=2007 |accessdate=2008-09-10}} </ref>. In principle, this mechanism also has higher quantum efficiency in producing white light. |
|||
There are several types of multi-colored white LEDs: [[di-]], [[tri-]], and [[tetrachromatic]] white LEDs. Several key factors that play among these different approaches include color [[stability]], [[color rendering index|color rendering]] capability, and [[luminous efficacy]]. Often higher efficacy will mean lower color rendering, presenting a trade off between the luminous efficiency and color rendering. For example, the dichromatic white LEDs have the best luminous efficiency (120 lm/W), but the lowest color rendering capability. Oppositely although [[tetrachromatic]] white LEDs have excellent color rendering capability, they often have poor luminous efficiency. Trichromatic white LEDs are in between, having both good luminous efficiency (>70 lm/W) and fair color rendering capability. |
|||
What multi-color LEDs offer is not merely another solution of producing white light, but is a whole new technique of producing light of different colors. In principle, all [[Colors#Color_perception|perceivable colors]] can be produced by mixing different amounts of three primary colors, and this makes it possible to produce precise dynamic color control as well. As more effort is devoted to investigating this technique, multi-color LEDs should have profound influence on the fundamental method which we use to produce and control light color. However, before this type of LED can truly play a role on the market, several technical problems need to be solved. These certainly include that this type of LED's emission power decays [[exponentially]] with increasing temperature,<ref>{{cite news |
|||
| title = E. Fred Schubert and Jong Kyu Kim |
|||
| publish = Science 308, 1274 |
|||
| date = 2005}}</ref> |
|||
resulting in a substantial change in color stability. Such problem is not acceptable for industrial usage. Therefore, many new package designs aiming to solve this problem have been proposed, and their results are being reproduced by researchers and scientists. |
|||
====Phosphor based LEDs==== |
|||
[[Image:White LED.png|thumb|right|350px|Spectrum of a “white” LED clearly showing blue light which is directly emitted by the GaN-based LED (peak at about 465 nm) and the more broadband [[Stokes shift|Stokes-shifted]] light emitted by the Ce<sup>3+</sup>:YAG phosphor which emits at roughly 500–700 nm.]] |
|||
This method involves [[coating]] an LED of one color (mostly blue LED made of InGaN) with [[phosphor]] of different colors to produce white light, the resultant LEDs are called '''phosphor based white LEDs'''. A fraction of the blue light undergoes the [[Stokes shift]] being transformed from shorter wavelengths to longer. Depending on the color of the original LED, phosphors of different colors can be employed. If several phosphor layers of distinct colors are applied, the emitted spectrum is broadened, effectively increasing the [[Color Rendering Index|color rendering index]] (CRI) value of a given LED. |
|||
Phosphor based LEDs have a lower efficiency than normal LEDs due to the heat loss from the Stokes shift and also other phosphor-related degradation issues. However, the phosphor method is still the most popular technique for manufacturing [[Led#High_power LEDs|high intensity]] white LEDs. The design and production of a light source or light fixture using a monochrome emitter with phosphor conversion is simpler and cheaper than a complex [[Led#RGB_Systems|RGB]] system, and the majority of high intensity white LEDs presently on the market are manufactured using phosphor light conversion. |
|||
The largest issue for the efficacy is the seemingly unavoidable Stokes energy loss. However, much effort is being spent on optimizing these devices to higher light output and higher operation temperatures. The efficiency can for instance be increased by adapting better package design or by using a more suitable type of phosphor. [[Philips Lumileds Lighting Company|Philips Lumileds]] patented conformal coating process addresses for instance the issue of varying phosphor thickness, giving the white LEDs a more homogeneous white light. With development ongoing the efficacy is generally increased with every new product announcement. |
|||
Technically the phosphor based white LEDs encapsulate InGaN blue LEDs inside of a phosphor coated epoxy. A common yellow phosphor material is [[cerium]]-[[Doping (Semiconductors)|doped]] [[YAG|yttrium aluminum garnet]] (Ce<sup>3+</sup>:YAG). |
|||
White LEDs can also be made by [[coating]] near [[ultraviolet]] (NUV) emitting LEDs with a mixture of high efficiency [[europium]]-based red and blue emitting phosphors plus green emitting copper and aluminum doped zinc sulfide (ZnS:Cu, Al). This is a method analogous to the way [[fluorescent lamp]]s work. However, the ultraviolet light causes [[photodegradation]] to the [[epoxy resin]] and many other materials used in LED packaging, causing manufacturing challenges and shorter lifetimes. This method is less efficient than the blue LED with YAG:Ce phosphor, as the [[Stokes shift]] is larger and more energy is therefore converted to heat, but yields light with better spectral characteristics, which render color better. Due to the higher radiative output of the ultraviolet LEDs than of the blue ones, both approaches offer comparable brightness. Another concern is that UV light may leak from a malfunctioning light source and cause harm to human eyes or skin. |
|||
The newest method used to produce white light LEDs uses no phosphors at all and is based on [[wiktionary:homoepitaxially|homoepitaxially]] grown [[zinc selenide]] (ZnSe) on a ZnSe substrate which simultaneously emits blue light from its active region and yellow light from the substrate.{{Fact|date=August 2008}} |
|||
=== Efficiency and operational parameters=== |
|||
Typical indicator LEDs are designed to operate with no more than 30–60 [[milliwatt]]s (mW) of electrical power. Around 1999, [[Philips Lumileds Lighting Company|Philips Lumileds]] introduced power LEDs capable of continuous use at one [[watt]] (W). These LEDs used much larger semiconductor die sizes to handle the large power inputs. Also, the semiconductor dies were mounted onto metal slugs to allow for heat removal from the LED die. |
|||
One of the key advantages of LED-based lighting is its high efficiency, as measured by its light output per unit power input. White LEDs quickly matched and overtook the efficiency of standard incandescent lighting systems. In 2002, [[Philips Lumileds Lighting Company|Lumileds]] made five-watt LEDs available with a [[luminous efficacy]] of 18–22 [[lumen (unit)|lumens]] per watt (lm/W). For comparison, a conventional 60–100 W incandescent lightbulb produces around 15 lm/W, and standard fluorescent lights produce up to 100 lm/W. (The [[luminous efficacy]] article discusses these comparisons in more detail.) |
|||
In September 2003, a new type of blue LED was demonstrated by the company [[Cree Inc.|Cree, Inc.]] to provide 24 mW at 20 [[milliampere]]s (mA). This produced a commercially packaged white light giving 65 lm/W at 20 mA, becoming the brightest white LED commercially available at the time, and more than four times as efficient as standard incandescents. In 2006 they demonstrated a prototype with a record white LED luminous efficacy of 131 lm/W at 20 mA. Also, [[Seoul Semiconductor]] has plans for 135 lm/W by 2007 and 145 lm/W by 2008, which would be approaching an order of magnitude improvement over standard incandescents and better even than standard fluorescents.<ref>{{cite news |url=http://www.engadget.com/2006/12/12/seoul-semiconductor-squeezes-240-lumens-into-brightest-led/ |title=Seoul Semiconductor squeezes 240 lumens into "brightest" LED |publisher=engadget |date=[[December 12]] [[2006]] |accessdate=2007-08-13}}</ref> [[Nichia Corporation]] has developed a white light LED with luminous efficacy of 150 lm/W at a forward current of 20 mA.<ref>{{cite news |url=http://techon.nikkeibp.co.jp/english/NEWS_EN/20061221/125713/ |title=Nichia Unveils White LED with 150 lm/W Luminous Efficiency |publisher=Tech-On! |date=[[December 21]] [[2006]] |accessdate=2007-08-13}}</ref> |
|||
It should be noted that high-power (≥ 1 W) LEDs are necessary for practical general lighting applications. Typical operating currents for these devices begin at 350 mA. The highest efficiency high-power white LED is claimed<ref>Datasheet is not sufficient to confirm the claim, comparing Philips LXHL-LW6C and OSRAM LUW W5AM-LXLY-6P7R </ref> by Philips Lumileds Lighting Co. with a luminous efficacy of 115 lm/W (350 mA). |
|||
=== Failure modes === |
|||
The most common way for LEDs (and [[diode laser]]s) to fail is the gradual lowering of light output and loss of efficiency. Sudden failures, however rare, can occur as well. Early red LEDs were notable for their short lifetime. |
|||
*'''[[Nucleation]] and growth of [[dislocation]]s''' is a known mechanism for degradation of the active region, where the radiative recombination occurs. This requires a presence of an existing defect in the crystal and is accelerated by heat, high current density, and emitted light. [[Gallium arsenide]] and [[aluminium gallium arsenide]] are more susceptible to this mechanism than [[gallium arsenide phosphide]] and [[indium phosphide]]. Due to different properties of the active regions, [[gallium nitride]] and [[indium gallium nitride]] are virtually insensitive to this kind of defect. |
|||
*'''[[Electromigration]]''' caused by high current density can move atoms out of the active regions, leading to emergence of dislocations and point defects, acting as nonradiative recombination centers and producing heat instead of light. |
|||
*'''[[Ionizing radiation]]''' can lead to the creation of defects as well, which leads to issues with [[radiation hardening]] of circuits containing LEDs (e.g., in [[optoisolator]]s) |
|||
* '''Differentiated phosphor degeneration.''' The different phosphors used in white LEDs tend to degrade with heat and age, but at different rates causing changes in the produced light color, for example, purple and pink LEDs often use an organic phosphor formulation which may degrade after just a few hours of operation causing a major shift in output color<ref>{{cite web |
|||
| url = http://ledmuseum.candlepower.us/ledpink.htm |
|||
| title = Candlepower Pink LED Reviews |
|||
| accessdate = 2008-09-19 |
|||
}}</ref>. |
|||
* '''Metal diffusion''' caused by high electrical currents or voltages at elevated temperatures can move metal atoms from the electrodes into the active region. Some materials, notably [[indium tin oxide]] and [[silver]], are subject to electromigration which causes leakage current and non radiative recombination along the chip edges. In some cases, especially with GaN/InGaN diodes, a [[barrier metal]] layer is used to hinder the electromigration effects. |
|||
*'''[[Short circuits]]''' Mechanical stresses, high currents, and corrosive environment can lead to formation of [[whisker (metallurgy)|whiskers]], causing short circuits. |
|||
*'''[[Thermal runaway]]''' Nonhomogenities in the substrate, causing localized loss of [[thermal conductivity]], can cause thermal runaway where heat causes damage which causes more heat etc. Most common ones are voids caused by incomplete [[soldering]], or by electromigration effects and [[Kirkendall voiding]]. |
|||
*'''[[Current crowding]]''', non-homogenous distribution of the current density over the junction. This may lead to creation of localized [[hot spot]]s, which poses risk of [[thermal runaway]]. |
|||
*'''Epoxy degradation''' Some materials of the plastic package tend to yellow when subjected to heat, causing partial absorption (and therefore loss of efficiency) of the affected wavelengths. |
|||
* '''[[Thermal stress]]''' Sudden failures are most often caused by thermal stresses. When the [[epoxy resin]] package reaches its [[glass transition temperature]], it starts rapidly expanding, causing mechanical stresses on the semiconductor and the [[wire bonding|bonded]] contact, weakening it or even tearing it off. Conversely, very low temperatures can cause cracking of the packaging. |
|||
*'''[[Electrostatic discharge]]''' (ESD) may cause immediate failure of the semiconductor junction, a permanent shift of its parameters, or latent damage causing increased rate of degradation. LEDs and lasers grown on [[sapphire]] substrate are more susceptible to ESD damage. |
|||
*'''[[Reverse bias]]''' Although the LED is based on a diode junction and is nominally a rectifier, the reverse-breakdown mode for some types can occur at very low voltages and essentially any reverse bias causes instantaneous failure. |
|||
=== Organic light-emitting diodes (OLEDs) === |
|||
{{main|Organic light-emitting diode}} |
|||
If the emitting layer material of the LED is an [[organic compound]], it is known as an Organic Light Emitting Diode ([[Organic light-emitting diode|OLED]]). To function as a semiconductor, the organic emitting material must have [[conjugated system|conjugated pi bonds]]. The emitting material can be a small organic [[molecule]] in a [[crystal]]line [[phase (matter)|phase]], or a [[polymer]]. Polymer materials can be flexible; such LEDs are known as PLEDs or FLEDs. |
|||
Compared with regular LEDs, OLEDs are lighter, and polymer LEDs can have the added benefit of being flexible. Some possible future applications of OLEDs could be: |
|||
* Inexpensive, flexible displays |
|||
* Light sources |
|||
* Wall decorations |
|||
* Luminous [[cloth]] |
|||
OLEDs have been used to produce visual displays for portable electronic devices such as cellphones, digital cameras, and MP3 players. Larger displays have been demonstrated, but their life expectancy is still far too short (<1,000 hours) to be practical. |
|||
Today, OLEDs operate at substantially lower efficiency than inorganic (crystalline) LEDs. The best luminous efficacy of an OLED so far is about 68 lm/W {{Fact|date=June 2008}}. |
|||
===Experimental technologies=== |
|||
====Quantum Dot LEDs==== |
|||
A new technique developed by Michael Bowers, a graduate student at [[Vanderbilt University]] in Nashville, involves coating a blue LED with [[quantum dots]] that glow white in response to the blue light from the LED. This technique produces a warm, yellowish-white light similar to that produced by [[incandescent bulb]]s.<ref>{{cite news |
|||
| title = Accidental Invention Points to End of Light Bulbs |
|||
| publisher =LiveScience.com |
|||
| date = [[October 21]] [[2005]] |
|||
| url = http://www.livescience.com/technology/051021_nano_light.html |
|||
| accessdate = 2007-01-24 }}</ref> |
|||
[[Quantum Dots]] are [[semiconductor]] nanocrystals that possess unique optical properties.<ref name=MITqdot2002>[http://web.mit.edu/newsoffice/2002/dot.html Quantum-dot LED may be screen of choice for future electronics] Massachusetts Institute of Technology News Office,December 18, 2002</ref> Their emission color can be tuned from the visible throughout the infrared spectrum. This allows quantum dot LEDs to create almost any color on the [[International Commission on Illumination|CIE]] diagram. This provides more color options and better color rendering white LEDs. Quantum dot LEDs are available in the same package types as traditional [[phosphor]] based LEDs. |
|||
====Research on DNA==== |
|||
At the [[University of Cincinnati]] the [[DNA]] in salmon sperm has recently been discovered to amplify the effects and quality of an LED light[http://www.uc.edu/news/NR.asp?id=7089] [http://www.theregister.co.uk/2007/09/18/led_salmon_dna_product_enhancement/]. |
|||
==Considerations in use== |
|||
===Electrical polarity=== |
|||
[[Image:Uvled_highres_macro.jpg|thumb|right|Close-up of a typical LED in its case, showing the internal structure.]] |
|||
[[Image:+- of LED.svg|thumb|right]] |
|||
Unlike [[incandescent light bulb]]s, which light up regardless of the electrical [[Polarity (physics)|polarity]], LEDs will only light with correct electrical polarity. When the voltage across the ''p-n junction'' is in the correct direction, a significant current flows and the device is said to be ''forward-biased''. If the voltage is of the wrong polarity, the device is said to be ''reverse biased'', very little current flows, and no light is emitted. LEDs can be operated on an [[alternating current]] voltage, but they will only light with positive voltage, causing the LED to turn on and off at the frequency of the AC supply. |
|||
Most LEDs have low [[breakdown voltage|reverse breakdown voltage]] ratings, so they will also be damaged by an applied reverse voltage above this threshold. If it is desired to drive the LED directly from an AC supply of more than the reverse breakdown voltage then it may be protected by placing a diode (or another LED) in [[Antiparallel (electronics)|inverse parallel]]. |
|||
The manufacture will normally advise on the correct way to determine the polarity of the LED in the product datasheet. However, these methods may also be used: |
|||
{|class="wikitable" |
|||
|- |
|- |
||
|'''sign:''' |
|||
|style="vertical-align: middle; border-top: 1px solid gray;" | For your effort in saving [[La Cala Resort]] from being deleted -- job well done! [[User:Ecoleetage|Ecoleetage]] ([[User talk:Ecoleetage|talk]]) 18:05, 10 October 2008 (UTC) |
|||
|'''+''' |
|||
|'''-''' |
|||
|- |
|||
|terminal: |
|||
|anode (A) |
|||
|cathode (K) |
|||
|- |
|||
|leads: |
|||
|long |
|||
|short |
|||
|- |
|||
|exterior: |
|||
|round |
|||
|flat |
|||
|- |
|||
|interior: |
|||
|small |
|||
|large |
|||
|- |
|||
|wiring: |
|||
|red |
|||
|black |
|||
|- |
|||
|*marking: |
|||
|none |
|||
|stripe |
|||
|- |
|||
|*pin: |
|||
|1 |
|||
|2 |
|||
|- |
|||
|*[[Printed circuit board|PCB]]: |
|||
|round |
|||
|square |
|||
|- |
|||
|*Die placement: |
|||
|connector |
|||
|cup |
|||
|} |
|} |
||
(*)Less reliable methods of determining polarity |
|||
It is strongly recommended to apply a safe voltage and observe the illumination as a test regardless of what method is used to determine the polarity. |
|||
===Power sources=== |
|||
{{Citations missing|date=August 2008}} |
|||
The voltage versus current characteristics of an LED are much like any [[diode]]. Current is approximately an exponential function of voltage, so a small voltage change results in a large change in current. This can result either in a unlit LED or a current above the maximum rating, potentially destroying the LED; as the LED heats, its voltage drop decreases, further increasing current. Consequently, LEDs cannot connect directly to constant-voltage sources. A series resistor is a very simple and common way to stabilize the LED current, but wastes energy in the resistor. A [[constant current]] regulator is commonly used. Low drop-out (LDO) constant current regulators also allow the total LED string voltage to be a higher percentage of the power supply voltage, resulting in improved efficiency and reduced power use. Switching-type converters are used in some LED flashlights, stabilizing light output over a wide range of battery voltages and increasing the useful life of the batteries. |
|||
====Low power LEDs==== |
|||
Miniature indicator LEDs are normally driven from low voltage DC via a current limiting [[resistor]]. Currents of 2 mA, 10 mA and 20 mA are common. |
|||
Sub-mA indicators may be made by driving ultrabright LEDs at very low current. Efficacy tends to reduce at low currents{{Fact|date=August 2008}}, but indicators running on 100 μA are still practical. The cost of ultrabrights is higher than 2 mA indicator LEDs. |
|||
Multiple LEDs are normally operated in parallel strings of series LEDs, with the total LED voltage typically adding up to around two-thirds of the supply voltage, with [[resistor]] [[current]] control for each string. |
|||
In disposable [[button cell|coin cell]] powered keyring type LED lights, the resistance of the cell itself is usually the only current limiting device. The cell should not therefore be replaced with a lower resistance type. |
|||
LEDs can be purchased with built in series resistors. These can save [[printed circuit board]] space and are especially useful when building [[prototype]]s or populating a PCB in a way other than its designers intended. However, the resistor value is set at the time of manufacture, removing one of the key methods of setting the LED's intensity. |
|||
Alphanumeric LEDs use the same drive strategy as indicator LEDs, the only difference being the larger number of channels, each with its own resistor. Seven-segment and starburst LED arrays are available in both common-anode or common-cathode form. |
|||
Finally, LEDs can be run from a single cell by use of a constant current switched mode invertor. The extra expense makes this option unpopular. |
|||
===Lighting LEDs on mains=== |
|||
LEDs by their very nature, require constant current with low voltage, as oppose to the electrical grid which supplies high voltage with AC. |
|||
A CR dropper followed by full wave rectification is the usual ballast with series-parallel LED clusters. A single series string minimises dropper losses, while paralleled strings increase reliability. In practice usually three strings or more are used. |
|||
Operation on square wave and modified sine wave (MSW) sources, such as many invertors, causes heavily increased resistor [[dissipation]] in CR droppers, and LED ballasts designed for sine wave use tend to burn on non-sine waveforms. The non-sine waveform also causes high peak LED currents, heavily shortening LED life. An inductor & rectifier makes a more suitable ballast for such use, and other options are also possible. |
|||
Multiple LEDs can be connected in [[Series and parallel circuits|series]] with a single current limiting resistor provided the source voltage is greater than the sum of the individual LED threshold voltages. [[Series and parallel circuits|Parallel]] operation is also possible but can be more problematic. Parallel LEDs must have closely matched forward voltages (Vf) in order to have equal branch currents and, therefore, equal light output. Variations in the manufacturing process can make it difficult to obtain satisfactory operation when connecting some types of LEDs in parallel.<ref>{{cite web |
|||
|url=http://www.nichia.co.jp/specification/appli/electrical.pdf |
|||
|format=PDF |
|||
|title=Electrical properties of GaN LEDs & Parallel connections |
|||
|accessdate=2007-08-13 |
|||
|work=Application Note |publisher=Nichia}}</ref> |
|||
To increase efficiency (or to allow intensity control without the complexity of a [[Digital-to-analog converter|DAC]]), the power may be applied periodically or intermittently; so long as the flicker rate is greater than the human [[flicker fusion threshold]], the LED will appear to be continuously lit. |
|||
=== Advantages of using LEDs === |
|||
*'''Efficacy:''' LEDs produce more light per watt than incandescent bulbs; this is useful in battery powered or energy-saving devices.<ref>{{cite web|url=http://www.netl.doe.gov/ssl/usingLeds/general_illumination_efficiency_comparison.htm|title=Solid-State Lighting: Comparing LEDs to Traditional Light Sources}}</ref> |
|||
*'''Color:''' LEDs can emit light of an intended color without the use of color filters that traditional lighting methods require. This is more efficient and can lower initial costs. |
|||
*'''Size:''' LEDs can be very small (>2 mm<sup>2</sup>) and are easily populated onto printed circuit boards. |
|||
*'''On/Off time:''' LEDs light up very quickly. A typical red indicator LED will achieve full brightness in microseconds<ref>Philips Lumileds technical datasheet DS23 for the Luxeon Star states "less than 100ns".</ref>. LEDs used in communications devices can have even faster response times. |
|||
*'''Cyceling:''' LEDs are ideal for use in applications that are subject to frequent on-off cycling, unlike fluorescent lamps that burn out more quickly when cycled frequently, or [[HID lamp]]s that require a long time before restarting. |
|||
*'''Dimming:''' LEDs can very easily be [[Dimmer|dimmed]] either by [[Pulse-width modulation]] or lowering the forward current. |
|||
*'''Slow faliure:''' LEDs mostly fail by dimming over time, rather than the abrupt burn-out of incandescent bulbs.<ref>{{cite web|url=http://www.netl.doe.gov/ssl/usingLeds/general_illumination_life_depreciation.htm|title=Solid-State Lighting: Lumen Depreciation}}</ref> |
|||
* '''Lifetime:''' LEDs can have a relatively long useful life. One report estimates 35,000 to 50,000 hours of useful life, though time to complete failure may be longer.<ref>http://www.netl.doe.gov/ssl/PDFs/lifetimeWhiteLEDs_aug16_r1.pdf</ref> Fluorescent tubes typically are rated at about 30,000 hours, and incandescent light bulbs at 1,000–2,000 hours.{{Fact|date=December 2007}} |
|||
* '''Shock resistance:'''LEDs, being solid state components, are difficult to damage with external shock, unlike fluorescent and incandescent bulbs which are fragile. |
|||
* '''Focus:''' The solid package of the LED can be designed to focus its light. Incandescent and fluorescent sources often require an external reflector to collect light and direct it in a usable manner. |
|||
*'''Toxicity:''' LEDs do not contain [[mercury (element)|mercury]], unlike [[fluorescent lamp]]s. |
|||
[[Image:Verschiedene LEDs.jpg|thumb|center|750px|LEDs are produced in an array of shapes and sizes. The 5 mm cylindrical package (red, fifth from the left) is the most common, estimated at 80% of world production. The color of the plastic lens is often the same as the actual color of light emitted, but not always. For instance, purple plastic is often used for [[infrared]] LEDs, and most blue devices have clear housings. There are also LEDs in [[Surface-mount technology|extremely tiny packages]], such as those found on [[blinky (novelty)|blinkies]] and on cell phone keypads. (not shown).]] |
|||
=== Disadvantages of using LEDs === |
|||
*'''High price:''' LEDs are currently more expensive, price per lumen, on an initial capital cost basis, than more conventional lighting technologies. The additional expense partially stems from the relatively low lumen output and the drive circuitry and power supplies needed. However, when considering the total cost of ownership (including energy and maintenance costs), LEDs far surpass incandescent or halogen sources and begin to threaten compact fluorescent lamps{{Fact|date=July 2008}}. |
|||
*'''Temperature dependence:''' LED performance largely depends on the ambient temperature of the operating environment. Over-driving the LED in high ambient temperatures may result in overheating of the LED package, eventually leading to device failure. Adequate [[heat sink|heat-sinking]] is required to maintain long life. This is especially important when considering automotive, medical, and military applications where the device must operate over a large range of temperatures, and is required to have a low failure rate. |
|||
*'''Voltage sensitivity:''' LEDs must be supplied with the voltage above the threshold and a current below the rating. This can involve series resistors or current-regulated power supplies.<ref>[http://www.ledmuseum.org/ The Led Museum]</ref> |
|||
*'''Light quality:''' Most [[#White LEDs|white LEDs]] have spectra that differ significantly from a [[black body]] radiator like the sun or an incandescent light. The spike at 460 nm and dip at 500 nm can cause the color of objects to be [[color vision|perceived differently]] under LED illumination than sunlight or incandescent sources, due to [[metamerism (color)|metamerism]],<ref>{{cite web | url = http://www.jimworthey.com/jimtalk2006feb.html | title = How White Light Works | author = James A. Worthey | work = LRO Lighting Research Symposium, Light and Color | accessdate = 2007-10-06}}</ref> red surfaces being rendered particularly badly by typical phosphor based LEDs white LEDs. However, the color rendering properties of common fluorescent lamps are often inferior to what is now available in state-of-art white LEDs. |
|||
*'''Area light source:''' LEDs do not approximate a “point source” of light, so cannot be used in applications needing a spherical light field. LEDs are not capable of providing divergence below a few degrees. This is contrasted with lasers, which can produce beams with divergences of 0.2 degrees or less.<ref>Hecht, E: "Optics", Fourth Edition, page 591. Addison Wesley, 2002.</ref> |
|||
*'''Blue Hazzard:''' There is increasing concern that [[#Ultraviolet and blue LEDs|blue LEDs]] and [[#White LEDs|white LEDs]] are now capable of exceeding safe limits of the so-called [[blue-light hazard]] as defined in eye safety specifications such as ANSI/IESNA RP-27.1-05: Recommended Practice for Photobiological Safety for Lamp and Lamp Systems.<ref>{{cite news |url=http://texyt.com/bright+blue+leds+annoyance+health+risks |title=Blue LEDs: A health hazard? |date=[[January 15]] [[2007]] |publisher=texyt.com |accessdate=2007-09-03}}</ref><ref>{{cite web|title=Light Impacts: Science News Online, [[May 27]], [[2006]]|url=http://www.sciencenews.org/articles/20060527/bob9.asp}} 071214 sciencenews.org</ref> |
|||
*'''Blue pollution:''' Because [[#White LEDs|white LEDs]] emit much more blue light than conventional outdoor light sources such as [[Sodium vapor lamp|high-pressure sodium lamps]], the strong wavelength dependence of [[Rayleigh scattering]] means that LEDs can cause more [[light pollution]] than other light sources. It is therefore very important that LEDs are fully shielded when used outdoors. Compared to [[Sodium vapor lamp|low-pressure sodium lamps]], which emit at 589.3nm, the 460 nm emission spike of white and blue LEDs is scattered about 2.7 times more by the Earth's atmosphere. LEDs should not be used for outdoor lighting near astronomical observatories. |
|||
==Types== |
|||
The main types of LEDs are miniature, high power devices and custom designs such as alphanumeric or multi-color. |
|||
===Miniature LEDs=== |
|||
[[Image:LEDs 8 5 3mm.JPG|thumb|Different sized LEDs. 8 mm, 5 mm and 3 mm, with a wooden match to compare the sizes.]] |
|||
These are mostly single-die LEDs used as indicators, and they come in various-size packages: |
|||
* surface mount |
|||
* 2 mm |
|||
* 3 mm (T1) |
|||
* 5 mm (T1³⁄₄) |
|||
* 10 mm |
|||
* Other sizes are also available, but less common. |
|||
Common package shapes: |
|||
*Round, dome top |
|||
*Round, flat top |
|||
*Rectangular, flat top (often seen in LED bar-graph displays) |
|||
*Triangular or square, flat top |
|||
The encapsulation may also be clear or semi opaque to improve contrast and viewing angle. |
|||
There are three main categories of miniature single die LEDs: |
|||
* Low current — typically rated for 2 mA at around 2 V (approximately 4 mW consumption). |
|||
* Standard — 20 mA LEDs at around 2 V (approximately 40 mW) for red, orange, yellow & green, and 20 mA at 4–5 V (approximately 100 mW) for blue, violet and white. |
|||
* Ultra-high output — 20 mA at approximately 2 V or 4–5 V, designed for viewing in direct sunlight. |
|||
====Five- and twelve-volt LEDs==== |
|||
These are ordinary miniature LEDs that incorporate a suitable series [[resistor]] for direct connection to a 5 V or 12 V supply. |
|||
====Flashing LEDs==== |
|||
Flashing LEDs are used as attention seeking indicators where it is desired to avoid the complexity of external electronics. Flashing LEDs resemble standard LEDs but they contain an integrated [[multivibrator]] circuit inside which causes the LED to flash with a typical period of one second. In diffused lens LEDs this is visible as a small black dot. Most flashing LEDs emit light of a single color, but more sophisticated devices can flash between multiple colors and even fade through a color sequence using RGB color mixing. |
|||
===High power LEDs=== |
|||
[[Image:2007-07-24 High-power light emiting diodes (Luxeon, Lumiled).jpg|thumb|High power LEDs from [[Philips Lumileds Lighting Company]] mounted on a star shaped heat sink]] |
|||
High power LEDs (HPLED) can be driven at more than one [[ampere]] of current and give out large amounts of light. Since overheating is destructive, the HPLEDs must be highly efficient to minimize excess heat; furthermore, they are often mounted on a heat sink to allow for heat dissipation. If the heat from a HPLED is not removed, the device will burn out in seconds. |
|||
A single HPLED can often replace an incandescent bulb in a flashlight, or be set in an array to form a powerful [[LED lamp]]. |
|||
LEDs have been developed that can run directly from mains power without the need for a DC converter. For each half cycle part of the LED diode emits light and part is dark, and this is reversed during the next half cycle. Current efficiency is 80 lm/W.<ref>{{cite web|url=http://www.ledsmagazine.com/news/3/11/14|title=Seoul Semiconductor launches AC LED lighting source Acriche |publisher=LEDS Magazine |accessdate=2008-02-17}}</ref> |
|||
===Multi-color LEDs=== |
|||
A “bi-color LED” is actually two different LEDs in one case. It consists of two dies connected to the same two leads but in opposite directions. Current flow in one direction produces one color, and current in the opposite direction produces the other color. Alternating the two colors with sufficient frequency causes the appearance of a blended third color. For example, a red/green LED operated in this fashion will color blend to produce a yellow appearance. |
|||
A “tri-color LED” is also two LEDs in one case, but the two LEDs are connected to separate leads so that the two LEDs can be controlled independently and lit simultaneously. A three-lead arrangement is typical with one commmon lead (anode or cathode). |
|||
RGB LEDs contain red, green and blue emitters, generally using a four-wire connection with one common lead (anode or cathode). |
|||
The Taiwanese LED manufacturer [http://www.everlight.com Everlight] has introduced a 3 watt |
|||
RGB package capable of driving each die at 1 watt. |
|||
===Alphanumeric LEDs=== |
|||
[[Image:LED DISP.JPG|thumb|right|200px|Old [[calculator]] LED display.]] |
|||
LED displays are available in [[seven-segment display|seven-segment]] and [[Starburst display|starburst]] format. Seven-segment displays handle all numbers and a limited set of letters. Starburst displays can display all letters. |
|||
Seven-segment LED displays were in widespread use in the 1970s and 1980s, but increasing use of [[liquid crystal display]]s, with their lower power consumption and greater display flexibility, has reduced the popularity of numeric and alphanumeric LED displays. |
|||
== LED applications == |
|||
=== List of LED applications === |
|||
The many application of LEDs are very diverse but fall into three major categories: Visual signal application where the light goes more or less directly from the LED to the human eye, to convey a message or meaning. [[Lighting|Illumination]] where LED light is reflected from object to give visual response of these objects. And finally LEDs are also used to generate light for measuring and interacting with processes that do not involve the human visual system. |
|||
==== Indicators and signs ==== |
|||
[[Image:LED bus destination displays.jpg|thumb|right|200px|LED destination displays on buses, one with a colored route number.]] |
|||
[[Image:Outdoor LED screen by Igors Jefimovs.jpg|thumb|left|200px|Outdoor 4 x 3 m large LED screen used as clock.]] |
|||
[[Image:LedSignal.JPG|thumb|[[Traffic light]] using LED]] |
|||
* Status indicators on a variety of equipment |
|||
* [[LED Panels]] used as stadium displays, large television displays, electronic billboards and dynamic decorative displays. |
|||
* [[Traffic light]]s and signals |
|||
* [[Exit sign]]s |
|||
* Thin, lightweight message displays at airports and railway stations, and as destination displays for trains, buses, trams, and ferries. |
|||
* Red or yellow LEDs are used in indicator and alphanumeric displays in environments where night vision must be retained: aircraft cockpits, submarine and ship bridges, astronomy observatories, and in the field, e.g. night time animal watching and military field use. |
|||
* Red, yellow, green, and blue LEDs can be used for [[model railroading]] applications |
|||
* In dot matrix arrangements for displaying messages. |
|||
* Because of their long life and fast switching times, LEDs have been used for automotive [[Automotive lighting#Center High Mount Stop Lamp (CHMSL)|high-mounted brake lights]] and truck and bus brake lights and turn signals for some time, but many high-end vehicles are now starting to use LEDs for their entire rear light clusters. Besides the gain in [[reliability]], this has styling advantages because LEDs are capable of forming much thinner lights than incandescent lamps with [[parabolic reflector]]s. The significant improvement in the time taken to light up (perhaps 0.5s faster than an incandescent bulb) improves safety by giving drivers more time to react. It has been reported that at normal highway speeds this equals one car length increased reaction time for the car behind. White LED headlamps are beginning to make an appearance. |
|||
* As a medium quality [[voltage reference]] in electronic circuits. The forward voltage drop (e.g., about 1.7 V for a normal red LED) can be used instead of a [[Zener diode]] in low-voltage regulators. Although LED forward voltage is much more current-dependent than a good Zener, Zener diodes are not available below voltages of about 3 V. |
|||
* Glowlights, as a more expensive but longer lasting and reusable alternative to [[Glowsticks]]. |
|||
* [[Lumalive]], a photonic [[textile]] |
|||
* [[Emergency vehicle lighting]] |
|||
* LED-based [[Christmas lights]] available in different colors and with low energy consumption. |
|||
* LED-modules provide LEDs in a more usable form to people with less knowledge of electronics and soldering: the actual LEDs are contained within in protective and mountable casing, and a lead enables connection to power supply, typically 12 volts. LED modules are available in a wide range of shapes, sizes and colors. |
|||
==== Lighting ==== |
|||
[[Image:LEDFlashlight.jpg|thumb|right|200px|Flashlights and lanterns that utilize white LEDs are becoming increasingly popular due to their durability and longer battery life.]] |
|||
[[Image:Hexagonal White LED module.jpg|thumb|White LED module]] |
|||
* Replacement light bulbs |
|||
*[[Flashlight]]s with low energy usage and high durability |
|||
* Lanterns |
|||
* Streetlights |
|||
* Large scale video displays |
|||
* Architectural lighting |
|||
* Light source for [[machine vision]] systems, requiring bright, focused, homogeneous and possibly [[Strobe light|strobed]] illumination. |
|||
* [[Vehicle]] lighting on cars, motorcycles and [[Bicycle lighting#LEDs|bicycle lights]] |
|||
* [[Backlight]]ing for [[LCD]] televisions and lightweight [[laptop]] displays. Using RGB LEDs increase the color [[gamut]] by as much as 45%. |
|||
* Light source for [[DLP]] projectors |
|||
* [[Stage light]]s using banks of RGB LEDs to easily change color and decrease heating from traditional stage lighting. |
|||
* Medical lighting where IR-radiation and high temperatures are unwanted. |
|||
* Strobe lights or camera flashes that operate at a safe, low voltage, as opposed to the 250+ volts commonly found in [[xenon]] flashlamp-based lighting. This is particularly applicable to cameras on mobile phones, where space is at a premium an bulky voltage-increasing circuitry is undesirable. |
|||
==== Non visual applications ==== |
|||
[[Image:LED panel and plants.jpg|thumb|right|200px|LED panel light source used in an experiment on [[plant]] growth. The findings of such experiments may be used to grow food in space on long duration missions.]] |
|||
* [[Grow lights]] using LEDs to increase [[photosynthesis]] in [[plants]]<ref>{{cite journal |
|||
|author = Goins, GD and Yorio, NC and Sanwo, MM and Brown, CS |
|||
|title = Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting |
|||
|journal = Journal of Experimental Botany |
|||
|year = 1997 |
|||
|volume = 48 |
|||
|pages = 1407 |
|||
|number = 7 |
|||
|keywords = photosynthesis;LED |
|||
|publisher = Soc Experiment Biol |
|||
|doi = 10.1093/jxb/48.7.1407 |
|||
}}</ref> |
|||
* [[Remote control]]s, such as for TVs and VCRs, often use [[infrared]] LEDs. |
|||
* Movement sensors, for example in [[Optical mouse|optical computer mice]]. The Nintendo [[Wii]]'s sensor bar uses [[infrared]] LEDs. |
|||
* In [[optical fiber]] and [[Free Space Optics]] communications. |
|||
* In [[pulse oximeter]]s for measuring [[oxygen saturation]] |
|||
* LED [[Light therapy|phototherapy]] for [[acne]] using blue or red LEDs has been proven to significantly reduce acne over a three-month period.{{Fact|date=February 2007}} |
|||
* Some flatbed scanners use arrays of RGB LEDs rather than the typical [[cold-cathode fluorescent lamp]] as the light source. Having independent control of three illuminated colors allows the scanner to calibrate itself for more accurate color balance, and there is no need for warm-up. Furthermore, its sensors only need be monochromatic, since at any one point in time the page being scanned is only lit by a single color of light. |
|||
* [[Sterilization]] of water and other substances using [[UV]] light.<ref name=water sterilization"/> |
|||
* [[Touchscreen|Touch sensing]]: Since LEDs can also be used as [[photodiode]]s, they can be used for both photo emission and detection. This could be used in for example a touch-sensing screen that register reflected light from a finger or [[stylus]]<ref>{{cite journal |
|||
|author=Dietz, Yerazunis, and Leigh |
|||
|title=Very Low-Cost Sensing and Communication Using Bidirectional LEDs |
|||
|year=2004 |
|||
|url=http://www.merl.com/publications/TR2003-035/ |
|||
}} |
|||
</ref>. |
|||
*[[Opto-isolator]]s use an LED combined with a [[photodiode]] or [[phototransistor]] to provide a signal path with electrical isolation between two circuits. This is especially useful in medical equipment where the signals from a low voltage [[sensor]] circuit (usually battery powered) in contact with a living organism must be electrically isolated from any possible electrical failure in a recording or monitoring device operating at potentially dangerous voltages. An optoisolator also allows information to be transferred between circuits not sharing a common ground potential. |
|||
=== Light sources for machine vision systems === |
|||
[[Image:WashDownRL4.jpg|thumb|right|200px|Light sources for [[machine vision]] systems.]] |
|||
[[Machine vision]] systems often require bright and homogeneous illumination, so features of interest are easier to process. |
|||
LEDs are often used to this purpose, and this field of application is likely to remain one of the major application areas until price drops low enough to make signaling and illumination applications more widespread. |
|||
LEDs constitute a nearly ideal light source for [[machine vision]] systems for several main reasons: |
|||
* Size of illuminated field is usually comparatively small and Vision systems or [[smart camera]] are quite expensive, so cost of LEDs is usually a minor concern, compared to signaling applications. |
|||
* LED elements tend to be small and can be placed with high density over flat or even shaped substrates (PCBs etc) so that bright and homogeneous sources can be designed which direct light from tightly controlled directions on inspected parts. |
|||
* LEDs often have or can be used with small, inexpensive lenses and diffusers, helping to achieve high light densities and very good lighting control and homogeneity. |
|||
* LEDs can be easily strobed (in the microsecond range and below) and synchronized; their power also has reached high enough levels that sufficiently high intensity can be obtained, allowing well lit images even with very short light pulses: this is often used in order to obtain crisp and sharp “still” images of quickly-moving parts. |
|||
* LEDs come in several different colors and wavelengths, easily allowing to use the best color for each application, where different color may provide better visibility of features of interest. Having a precisely known spectrum allows tightly matched filters to be used to separate informative bandwidth or to reduce disturbing effect of ambient light. |
|||
* LEDs usually operate at comparatively low working temperatures, simplifying heat management and dissipation, therefore allowing plastic lenses, filters and diffusers to be used. Waterproof units can also easily be designed, allowing for use in harsh or wet environments (food, beverage, oil industries). |
|||
* LED sources can be shaped in several main configurations (spot lights for reflective illumination; ring lights for coaxial illumination; back lights for contour illumination; linear assemblies; flat, large format panels; dome sources for diffused, omnidirectional illumination). |
|||
* Very compact designs are possible, allowing for small LED illuminators to be integrated within [[smart camera]]s and vision sensors. |
|||
== See also == |
|||
{{portalpar|Electronics|Nuvola_apps_ksim.png}} |
|||
* [[Nystagmus]] An eye condition in which sufferers have difficulty focusing on LED displays |
|||
* [[Photometry (optics)]] Main Photometry/Radiometry article—explains technical terms |
|||
* [[LED lamp]]—[[solid state lighting]] (SSL) |
|||
* [[Flashlight]] about the growing use of LED technology in Flashlights |
|||
* [[Blinky (novelty)|Blinkies]] |
|||
* [[Throwies]] |
|||
* [[LED circuit]] |
|||
* [[Nixie tube]] |
|||
* [[Light Up the World Foundation]] |
|||
* [[Lumalive]], a photonic [[textile]] |
|||
== References == |
|||
;Cited |
|||
{{reflist|2}} |
|||
;General |
|||
<div class="references-small"> |
|||
* [http://www.amazon.com/dp/3540665056/ Shuji Nakamura, Gerhard Fasol, Stephen J Pearton The Blue Laser Diode: The Complete Story, Springer Verlag, 2nd Edition ([[October 2]], 2000)] |
|||
* {{cite journal | last=Mills |first= Evan | title=The Specter of Fuel-Based Lighting | journal=Science | year=2005 | volume=308 | pages=1263–1264 | url= | doi=10.1126/science.1113090 | pmid=15919979 }} |
|||
* Moreno, I., "Spatial distribution of LED radiation," in The International Optical Design Conference, Proc. SPIE vol. 6342, 634216:1-7 (2006). |
|||
* {{cite web |last=Salisbury |first=David F. |url=http://exploration.vanderbilt.edu/news/news_quantumdot_led.htm |title=Quantum dots that produce white light could be the light bulb’s successor |work=Exploration—The Online Research Journal of Vanderbilt University |date=[[October 20]], [[2005]]}} (More details regarding the use of quantum dots as a phosphor for white LEDs.) |
|||
</div> |
|||
== External links == |
|||
{{Commons|LED|Light-emitting diode}} |
|||
* [http://www.netl.doe.gov/ssl/faqs.htm Solid State Lighting program at U.S. DOE] |
|||
* [http://www.tyndall.ie/gan Photonics Sources Group, Tyndall National Institute] GaN and other photonics research at the Tyndall National Institute, Ireland. |
|||
* [http://ieee.li/pdf/viewgraphs_lighting.pdf Solid State Lighting, Michael Shur - Rensselaer Polytechnic] |
|||
* [http://www.intl-lighttech.com/applications/led-lamps Applications notes about Discrete LEDs including basic driver circuits] |
|||
* [http://www.lsdiodes.com/tutorial LED Circuitry Tutorial] |
|||
* [http://www.led-calculator.com LED Resistor Calculator] |
|||
{{ArtificialLightSources}} |
|||
{{Display Technology}} |
|||
[[Category:Display technology]] |
|||
[[Category:Optical diodes]] |
|||
[[Category:Lighting]] |
|||
[[Category:Semiconductor devices]] |
|||
[[Category:1907 introductions]] |
|||
[[bn:লাইট এমিটিং ডায়োড]] |
|||
<tr><td><center>'''[[Wikipedia:Babel]]'''</center></td></tr> |
|||
[[bs:Svjetleća dioda]] |
|||
<tr><td>{{User en}}</td></tr> |
|||
[[bg:Светодиод]] |
|||
<tr><td>{{User de-3}}</td></tr> |
|||
[[ca:Díode LED]] |
|||
<tr><td>{{User es-1}}</td></tr> |
|||
[[cs:LED]] |
|||
<tr><td>{{User fr-1}}</td></tr> |
|||
[[da:Lysdiode]] |
|||
</table> |
|||
[[de:Leuchtdiode]] |
|||
[[et:Valgusdiood]] |
|||
[[el:Δίοδος Εκπομπής Φωτός]] |
|||
[[es:Diodo emisor de luz]] |
|||
[[eo:Diodo lumeliganta]] |
|||
[[eu:LED diodo]] |
|||
[[fr:Diode électroluminescente]] |
|||
[[gl:LED]] |
|||
[[ko:발광 다이오드]] |
|||
[[hr:Svjetleća dioda]] |
|||
[[id:Dioda cahaya]] |
|||
[[is:Ljóstvistur]] |
|||
[[it:LED]] |
|||
[[he:דיודה פולטת אור]] |
|||
[[ka:მანათობელი დიოდები]] |
|||
[[lt:Šviesos diodas]] |
|||
[[hu:Fénykibocsátó dióda]] |
|||
[[ml:എല്.ഇ.ഡി.]] |
|||
[[ms:Diod pemancar cahaya]] |
|||
[[nl:Led]] |
|||
[[ja:発光ダイオード]] |
|||
[[no:Lysdiode]] |
|||
[[nn:Lysdiode]] |
|||
[[pl:Dioda elektroluminescencyjna]] |
|||
[[pt:LED]] |
|||
[[ro:LED]] |
|||
[[ru:Светодиод]] |
|||
[[simple:Light-emitting diode]] |
|||
[[sk:LED]] |
|||
[[sl:Svetleča dioda]] |
|||
[[sr:Светлећа диода]] |
|||
[[fi:LED]] |
|||
[[sv:Lysdiod]] |
|||
[[th:ไดโอดเปล่งแสง]] |
|||
[[vi:Luxeon]] |
|||
[[tr:LED]] |
|||
[[tl:Duhandas na nagsasaboy ng liwanag]] |
|||
[[uk:Світлодіод]] |
|||
[[zh:發光二極管]] |
|||
Revision as of 23:05, 10 October 2008

Infrared and ultraviolet (UVA) LEDs are also available.
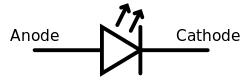

A light-emitting diode (LED) (Template:PronEng),[1] is a semiconductor diode that emits light when an electric current is applied in the forward direction of the device, as in the simple LED circuit. The effect is a form of electroluminescence where incoherent and narrow-spectrum light is emitted from the p-n junction.
LEDs are widely used as indicator lights on electronic devices and increasingly in higher power applications such as flashlights and area lighting. An LED is usually a small area (less than 1 mm2) light source, often with optics added to the chip to shape its radiation pattern and assist in reflection.[2][3] The color of the emitted light depends on the composition and condition of the semiconducting material used, and can be infrared, visible, or ultraviolet. Besides lighting, interesting applications include using UV-LEDs for sterilization of water and disinfection of devices,[4] and as a grow light to enhance photosynthesis in plants.[5]
History
Discovery and development
The first known report of a light-emitting solid-state diode was made in 1907 by the British experimenter H. J. Round of Marconi Labs.[6] Russian Oleg Vladimirovich Losev independently created the first LED in the mid 1920s; his research, though distributed in Russian, German and British scientific journals, was ignored,[7][8] and no practical use was made of the discovery for several decades. Rubin Braunstein of the Radio Corporation of America reported on infrared emission from gallium arsenide (GaAs) and other semiconductor alloys in 1955.[9] Braunstein observed infrared emission generated by simple diode structures using GaSb, GaAs, InP, and Ge-Si alloys at room temperature and at 77 K. In 1961, experimenters Bob Biard and Gary Pittman working at Texas Instruments,[10] found that gallium arsenide gave off infrared radiation when electric current was applied. Biard and Pittman were able to establish the priority of their work and received the patent for the infrared light-emitting diode.
The first practical visible-spectrum (red) LED was developed in 1962 by Nick Holonyak Jr., while working at General Electric Company. He later moved to the University of Illinois at Urbana-Champaign.[11] Holonyak is seen as the "father of the light-emitting diode".[12] M. George Craford, a former graduate student of Holonyak's, invented the first yellow LED and 10x brighter red and red-orange LEDs in 1972.[13]
Shuji Nakamura of Nichia Corporation of Japan demonstrated the first high-brightness blue LED based on InGaN borrowing on critical developments in GaN nucleation on sapphire substrates and the demonstration of p-type doping of GaN which were developed by I. Akasaki and H. Amano in Nagoya. In 1995, Alberto Barbieri at the Cardiff University Laboratory (GB) investigated the efficiency and reliability of high-brightness LEDs demonstrating very high result by using a transparent contact made of indium tin oxide (ITO) on (AlGaInP/GaAs) LED. The existence of blue LEDs and high efficiency LEDs quickly led to the development of the first white LED, which employed a Y3Al5O12:Ce, or "YAG", phosphor coating to mix yellow (down-converted) light with blue to produce light that appears white. Nakamura was awarded the 2006 Millennium Technology Prize for his invention.[14]
The development of LED technology has caused their efficiency and light output to increase exponentially, with a doubling occurring about every 36 months since the 1960s, in a similar way to Moore's law. The advances are generally attributed to the parallel development of other semiconductor technologies and advances in optics and material science. This trend is normally called Haitz's Law after Dr. Roland Haitz.
Practical use

The first commercial LEDs were commonly used as replacements for incandescent indicators, and in seven-segment displays, first in expensive equipment such as laboratory and electronics test equipment, then later in such appliances as TVs, radios, telephones, calculators, and even watches (see list of signal applications). These red LEDs were bright enough only for use as indicators, as the light output was not enough to illuminate an area. Later, other colors became widely available and also appeared in appliances and equipment. As the LED materials technology became more advanced, the light output was increased, while maintaining the efficiency and the reliability to an acceptable level, causing LEDs to become bright enough to be used for illumination, in various applications such as lamps and other lighting fixtures.
Most LEDs were made in the very common 5 mm T1³⁄₄ and 3 mm T1 packages, but with higher power, it has become increasingly necessary to shed excess heat in order to maintain reliability, so more complex packages adapted for efficient heat dissipation are becoming common. Packages for state-of-the-art high power LEDs bear little resemblance to early LEDs.
LED technology
Physical principles


Like a normal diode, the LED consists of a chip of semiconducting material impregnated, or doped, with impurities to create a p-n junction. As in other diodes, current flows easily from the p-side, or anode, to the n-side, or cathode, but not in the reverse direction. Charge-carriers—electrons and holes—flow into the junction from electrodes with different voltages. When an electron meets a hole, it falls into a lower energy level, and releases energy in the form of a photon.
The wavelength of the light emitted, and therefore its color, depends on the band gap energy of the materials forming the p-n junction. In silicon or germanium diodes, the electrons and holes recombine by a non-radiative transition which produces no optical emission, because these are indirect band gap materials. The materials used for the LED have a direct band gap with energies corresponding to near-infrared, visible or near-ultraviolet light.
LED development began with infrared and red devices made with gallium arsenide. Advances in materials science have made possible the production of devices with ever-shorter wavelengths, producing light in a variety of colors.
LEDs are usually built on an n-type substrate, with an electrode attached to the p-type layer deposited on its surface. P-type substrates, while less common, occur as well. Many commercial LEDs, especially GaN/InGaN, also use sapphire substrate.
Light extraction
The refractive index of most LED semiconductor materials is quite high, so in almost all cases the light from the LED is coupled into a much lower-index medium. The large index difference makes the reflection quite substantial (per the Fresnel coefficients). The produced light gets partially reflected back into the semiconductor, where it may be absorbed and turned into additional heat; this is usually one of the dominant causes of LED inefficiency. Often more than half of the emitted light is reflected back at the LED-package and package-air interfaces.
The reflection is most commonly reduced by using a dome-shaped (half-sphere) package with the diode in the center so that the outgoing light rays strike the surface perpendicularly, at which angle the reflection is minimized. Substrates that are transparent to the emitted wavelength, and backed by a reflective layer, increase the LED efficiency. The refractive index of the package material should also match the index of the semiconductor, to minimize back-reflection. An anti-reflection coating may be added as well.
The package may be colored, but this is only for cosmetic reasons or to improve the contrast ratio; the color of the packaging does not substantially affect the color of the light emitted.
Other strategies for reducing the impact of the interface reflections include designing the LED to reabsorb and reemit the reflected light (called photon recycling) and manipulating the microscopic structure of the surface to reduce the reflectance, by introducing random roughness, creating programmed moth eye surface patterns. Recently photonic crystal have also been used to minimize back-reflections.[15] In December 2007, scientists at Glasgow University claimed to have found a way to make LEDs more energy efficient, imprinting billions of holes into LEDs using a process known as nanoimprint lithography.[16]
Colors and Materials
Conventional LEDs are made from a variety of inorganic semiconductor materials, the following table shows the available colors with wavelength range, voltage drop and material:
Ultraviolet and blue LEDs
Blue LEDs are based on the wide band gap semiconductors GaN (gallium nitride) and InGaN (indium gallium nitride). They can be added to existing red and green LEDs to produce the impression of white light, though white LEDs today rarely use this principle.
The first blue LEDs were made in 1971 by Jacques Pankove (inventor of the gallium nitride LED) at RCA Laboratories.[18] However, these devices had too little light output to be of much practical use. In the late 1980s, key breakthroughs in GaN epitaxial growth and p-type doping by Isamu Akasaki and Hiroshi Amano (Nagoya, Japan)[19] ushered in the modern era of GaN-based optoelectronic devices. Building upon this foundation, in 1993 high brightness blue LEDs were demonstrated through the work of Shuji Nakamura at Nichia Corporation.[20]
By the late 1990s, blue LEDs had become widely available. They have an active region consisting of one or more InGaN quantum wells sandwiched between thicker layers of GaN, called cladding layers. By varying the relative InN-GaN fraction in the InGaN quantum wells, the light emission can be varied from violet to amber. AlGaN aluminium gallium nitride of varying AlN fraction can be used to manufacture the cladding and quantum well layers for ultraviolet LEDs, but these devices have not yet reached the level of efficiency and technological maturity of the InGaN-GaN blue/green devices. If the active quantum well layers are GaN, as opposed to alloyed InGaN or AlGaN, the device will emit near-ultraviolet light with wavelengths around 350–370 nm. Green LEDs manufactured from the InGaN-GaN system are far more efficient and brighter than green LEDs produced with non-nitride material systems.
With nitrides containing aluminium, most often AlGaN and AlGaInN, even shorter wavelengths are achievable. Ultraviolet LEDs in a range of wavelengths are becoming available on the market. Near-UV emitters at wavelengths around 375–395 nm are already cheap and often encountered, for example, as black light lamp replacements for inspection of anti-counterfeiting UV watermarks in some documents and paper currencies. Shorter wavelength diodes, while substantially more expensive, are commercially available for wavelengths down to 247 nm.[21] As the photosensitivity of microorganisms approximately matches the absorption spectrum of DNA, with a peak at about 260 nm, UV LEDs emitting at 250–270 nm are to be expected in prospective disinfection and sterilization devices. Recent research has shown that commercially available UVA LEDs (365 nm) are already effective disinfection and sterilization devices.[4]
Wavelengths down to 210 nm were obtained in laboratories using aluminium nitride.
While not an LED as such, an ordinary NPN bipolar transistor will emit violet light if its emitter-base junction is subjected to non-destructive reverse breakdown. This is easy to demonstrate by filing the top off a metal-can transistor (BC107, 2N2222 or similar) and biasing it well above emitter-base breakdown (≥ 20 V) via a current-limiting resistor.
White light LEDs
There are two ways of producing high intensity white-light using LEDs. One is to use individual LEDs that emit three primary colors[22] – red, green, and blue, and then mix all the colors to produce white light. The other is to use a phosphor material to convert monochromatic light from a blue or UV LED to broad-spectrum white light, much in the same way a fluorescent light bulb works.
RGB Systems

White light can be produced by mixing differently colored light, the most common method is to use red, green and blue (RGB). Hence the method is called multi-colored white LEDs (sometimes referred to as RGB LEDs). Because its mechanism is involved with sophisticated electro-optical design to control the blending and diffusion of different colors, this approach has rarely been used to mass produce white LEDs in the industry. Nevertheless this method is particularly interesting to many researchers and scientists because of the flexibility of mixing different colors [23]. In principle, this mechanism also has higher quantum efficiency in producing white light.
There are several types of multi-colored white LEDs: di-, tri-, and tetrachromatic white LEDs. Several key factors that play among these different approaches include color stability, color rendering capability, and luminous efficacy. Often higher efficacy will mean lower color rendering, presenting a trade off between the luminous efficiency and color rendering. For example, the dichromatic white LEDs have the best luminous efficiency (120 lm/W), but the lowest color rendering capability. Oppositely although tetrachromatic white LEDs have excellent color rendering capability, they often have poor luminous efficiency. Trichromatic white LEDs are in between, having both good luminous efficiency (>70 lm/W) and fair color rendering capability.
What multi-color LEDs offer is not merely another solution of producing white light, but is a whole new technique of producing light of different colors. In principle, all perceivable colors can be produced by mixing different amounts of three primary colors, and this makes it possible to produce precise dynamic color control as well. As more effort is devoted to investigating this technique, multi-color LEDs should have profound influence on the fundamental method which we use to produce and control light color. However, before this type of LED can truly play a role on the market, several technical problems need to be solved. These certainly include that this type of LED's emission power decays exponentially with increasing temperature,[24] resulting in a substantial change in color stability. Such problem is not acceptable for industrial usage. Therefore, many new package designs aiming to solve this problem have been proposed, and their results are being reproduced by researchers and scientists.
Phosphor based LEDs

This method involves coating an LED of one color (mostly blue LED made of InGaN) with phosphor of different colors to produce white light, the resultant LEDs are called phosphor based white LEDs. A fraction of the blue light undergoes the Stokes shift being transformed from shorter wavelengths to longer. Depending on the color of the original LED, phosphors of different colors can be employed. If several phosphor layers of distinct colors are applied, the emitted spectrum is broadened, effectively increasing the color rendering index (CRI) value of a given LED.
Phosphor based LEDs have a lower efficiency than normal LEDs due to the heat loss from the Stokes shift and also other phosphor-related degradation issues. However, the phosphor method is still the most popular technique for manufacturing high intensity white LEDs. The design and production of a light source or light fixture using a monochrome emitter with phosphor conversion is simpler and cheaper than a complex RGB system, and the majority of high intensity white LEDs presently on the market are manufactured using phosphor light conversion.
The largest issue for the efficacy is the seemingly unavoidable Stokes energy loss. However, much effort is being spent on optimizing these devices to higher light output and higher operation temperatures. The efficiency can for instance be increased by adapting better package design or by using a more suitable type of phosphor. Philips Lumileds patented conformal coating process addresses for instance the issue of varying phosphor thickness, giving the white LEDs a more homogeneous white light. With development ongoing the efficacy is generally increased with every new product announcement.
Technically the phosphor based white LEDs encapsulate InGaN blue LEDs inside of a phosphor coated epoxy. A common yellow phosphor material is cerium-doped yttrium aluminum garnet (Ce3+:YAG).
White LEDs can also be made by coating near ultraviolet (NUV) emitting LEDs with a mixture of high efficiency europium-based red and blue emitting phosphors plus green emitting copper and aluminum doped zinc sulfide (ZnS:Cu, Al). This is a method analogous to the way fluorescent lamps work. However, the ultraviolet light causes photodegradation to the epoxy resin and many other materials used in LED packaging, causing manufacturing challenges and shorter lifetimes. This method is less efficient than the blue LED with YAG:Ce phosphor, as the Stokes shift is larger and more energy is therefore converted to heat, but yields light with better spectral characteristics, which render color better. Due to the higher radiative output of the ultraviolet LEDs than of the blue ones, both approaches offer comparable brightness. Another concern is that UV light may leak from a malfunctioning light source and cause harm to human eyes or skin.
The newest method used to produce white light LEDs uses no phosphors at all and is based on homoepitaxially grown zinc selenide (ZnSe) on a ZnSe substrate which simultaneously emits blue light from its active region and yellow light from the substrate.[citation needed]
Efficiency and operational parameters
Typical indicator LEDs are designed to operate with no more than 30–60 milliwatts (mW) of electrical power. Around 1999, Philips Lumileds introduced power LEDs capable of continuous use at one watt (W). These LEDs used much larger semiconductor die sizes to handle the large power inputs. Also, the semiconductor dies were mounted onto metal slugs to allow for heat removal from the LED die.
One of the key advantages of LED-based lighting is its high efficiency, as measured by its light output per unit power input. White LEDs quickly matched and overtook the efficiency of standard incandescent lighting systems. In 2002, Lumileds made five-watt LEDs available with a luminous efficacy of 18–22 lumens per watt (lm/W). For comparison, a conventional 60–100 W incandescent lightbulb produces around 15 lm/W, and standard fluorescent lights produce up to 100 lm/W. (The luminous efficacy article discusses these comparisons in more detail.)
In September 2003, a new type of blue LED was demonstrated by the company Cree, Inc. to provide 24 mW at 20 milliamperes (mA). This produced a commercially packaged white light giving 65 lm/W at 20 mA, becoming the brightest white LED commercially available at the time, and more than four times as efficient as standard incandescents. In 2006 they demonstrated a prototype with a record white LED luminous efficacy of 131 lm/W at 20 mA. Also, Seoul Semiconductor has plans for 135 lm/W by 2007 and 145 lm/W by 2008, which would be approaching an order of magnitude improvement over standard incandescents and better even than standard fluorescents.[25] Nichia Corporation has developed a white light LED with luminous efficacy of 150 lm/W at a forward current of 20 mA.[26]
It should be noted that high-power (≥ 1 W) LEDs are necessary for practical general lighting applications. Typical operating currents for these devices begin at 350 mA. The highest efficiency high-power white LED is claimed[27] by Philips Lumileds Lighting Co. with a luminous efficacy of 115 lm/W (350 mA).
Failure modes
The most common way for LEDs (and diode lasers) to fail is the gradual lowering of light output and loss of efficiency. Sudden failures, however rare, can occur as well. Early red LEDs were notable for their short lifetime.
- Nucleation and growth of dislocations is a known mechanism for degradation of the active region, where the radiative recombination occurs. This requires a presence of an existing defect in the crystal and is accelerated by heat, high current density, and emitted light. Gallium arsenide and aluminium gallium arsenide are more susceptible to this mechanism than gallium arsenide phosphide and indium phosphide. Due to different properties of the active regions, gallium nitride and indium gallium nitride are virtually insensitive to this kind of defect.
- Electromigration caused by high current density can move atoms out of the active regions, leading to emergence of dislocations and point defects, acting as nonradiative recombination centers and producing heat instead of light.
- Ionizing radiation can lead to the creation of defects as well, which leads to issues with radiation hardening of circuits containing LEDs (e.g., in optoisolators)
- Differentiated phosphor degeneration. The different phosphors used in white LEDs tend to degrade with heat and age, but at different rates causing changes in the produced light color, for example, purple and pink LEDs often use an organic phosphor formulation which may degrade after just a few hours of operation causing a major shift in output color[28].
- Metal diffusion caused by high electrical currents or voltages at elevated temperatures can move metal atoms from the electrodes into the active region. Some materials, notably indium tin oxide and silver, are subject to electromigration which causes leakage current and non radiative recombination along the chip edges. In some cases, especially with GaN/InGaN diodes, a barrier metal layer is used to hinder the electromigration effects.
- Short circuits Mechanical stresses, high currents, and corrosive environment can lead to formation of whiskers, causing short circuits.
- Thermal runaway Nonhomogenities in the substrate, causing localized loss of thermal conductivity, can cause thermal runaway where heat causes damage which causes more heat etc. Most common ones are voids caused by incomplete soldering, or by electromigration effects and Kirkendall voiding.
- Current crowding, non-homogenous distribution of the current density over the junction. This may lead to creation of localized hot spots, which poses risk of thermal runaway.
- Epoxy degradation Some materials of the plastic package tend to yellow when subjected to heat, causing partial absorption (and therefore loss of efficiency) of the affected wavelengths.
- Thermal stress Sudden failures are most often caused by thermal stresses. When the epoxy resin package reaches its glass transition temperature, it starts rapidly expanding, causing mechanical stresses on the semiconductor and the bonded contact, weakening it or even tearing it off. Conversely, very low temperatures can cause cracking of the packaging.
- Electrostatic discharge (ESD) may cause immediate failure of the semiconductor junction, a permanent shift of its parameters, or latent damage causing increased rate of degradation. LEDs and lasers grown on sapphire substrate are more susceptible to ESD damage.
- Reverse bias Although the LED is based on a diode junction and is nominally a rectifier, the reverse-breakdown mode for some types can occur at very low voltages and essentially any reverse bias causes instantaneous failure.
Organic light-emitting diodes (OLEDs)
If the emitting layer material of the LED is an organic compound, it is known as an Organic Light Emitting Diode (OLED). To function as a semiconductor, the organic emitting material must have conjugated pi bonds. The emitting material can be a small organic molecule in a crystalline phase, or a polymer. Polymer materials can be flexible; such LEDs are known as PLEDs or FLEDs.
Compared with regular LEDs, OLEDs are lighter, and polymer LEDs can have the added benefit of being flexible. Some possible future applications of OLEDs could be:
- Inexpensive, flexible displays
- Light sources
- Wall decorations
- Luminous cloth
OLEDs have been used to produce visual displays for portable electronic devices such as cellphones, digital cameras, and MP3 players. Larger displays have been demonstrated, but their life expectancy is still far too short (<1,000 hours) to be practical.
Today, OLEDs operate at substantially lower efficiency than inorganic (crystalline) LEDs. The best luminous efficacy of an OLED so far is about 68 lm/W [citation needed].
Experimental technologies
Quantum Dot LEDs
A new technique developed by Michael Bowers, a graduate student at Vanderbilt University in Nashville, involves coating a blue LED with quantum dots that glow white in response to the blue light from the LED. This technique produces a warm, yellowish-white light similar to that produced by incandescent bulbs.[29]
Quantum Dots are semiconductor nanocrystals that possess unique optical properties.[30] Their emission color can be tuned from the visible throughout the infrared spectrum. This allows quantum dot LEDs to create almost any color on the CIE diagram. This provides more color options and better color rendering white LEDs. Quantum dot LEDs are available in the same package types as traditional phosphor based LEDs.
Research on DNA
At the University of Cincinnati the DNA in salmon sperm has recently been discovered to amplify the effects and quality of an LED light[1] [2].
Considerations in use
Electrical polarity


Unlike incandescent light bulbs, which light up regardless of the electrical polarity, LEDs will only light with correct electrical polarity. When the voltage across the p-n junction is in the correct direction, a significant current flows and the device is said to be forward-biased. If the voltage is of the wrong polarity, the device is said to be reverse biased, very little current flows, and no light is emitted. LEDs can be operated on an alternating current voltage, but they will only light with positive voltage, causing the LED to turn on and off at the frequency of the AC supply.
Most LEDs have low reverse breakdown voltage ratings, so they will also be damaged by an applied reverse voltage above this threshold. If it is desired to drive the LED directly from an AC supply of more than the reverse breakdown voltage then it may be protected by placing a diode (or another LED) in inverse parallel.
The manufacture will normally advise on the correct way to determine the polarity of the LED in the product datasheet. However, these methods may also be used:
| sign: | + | - |
| terminal: | anode (A) | cathode (K) |
| leads: | long | short |
| exterior: | round | flat |
| interior: | small | large |
| wiring: | red | black |
| *marking: | none | stripe |
| *pin: | 1 | 2 |
| *PCB: | round | square |
| *Die placement: | connector | cup |
(*)Less reliable methods of determining polarity
It is strongly recommended to apply a safe voltage and observe the illumination as a test regardless of what method is used to determine the polarity.
Power sources
This article needs additional citations for verification. (August 2008) |
The voltage versus current characteristics of an LED are much like any diode. Current is approximately an exponential function of voltage, so a small voltage change results in a large change in current. This can result either in a unlit LED or a current above the maximum rating, potentially destroying the LED; as the LED heats, its voltage drop decreases, further increasing current. Consequently, LEDs cannot connect directly to constant-voltage sources. A series resistor is a very simple and common way to stabilize the LED current, but wastes energy in the resistor. A constant current regulator is commonly used. Low drop-out (LDO) constant current regulators also allow the total LED string voltage to be a higher percentage of the power supply voltage, resulting in improved efficiency and reduced power use. Switching-type converters are used in some LED flashlights, stabilizing light output over a wide range of battery voltages and increasing the useful life of the batteries.
Low power LEDs
Miniature indicator LEDs are normally driven from low voltage DC via a current limiting resistor. Currents of 2 mA, 10 mA and 20 mA are common.
Sub-mA indicators may be made by driving ultrabright LEDs at very low current. Efficacy tends to reduce at low currents[citation needed], but indicators running on 100 μA are still practical. The cost of ultrabrights is higher than 2 mA indicator LEDs.
Multiple LEDs are normally operated in parallel strings of series LEDs, with the total LED voltage typically adding up to around two-thirds of the supply voltage, with resistor current control for each string.
In disposable coin cell powered keyring type LED lights, the resistance of the cell itself is usually the only current limiting device. The cell should not therefore be replaced with a lower resistance type.
LEDs can be purchased with built in series resistors. These can save printed circuit board space and are especially useful when building prototypes or populating a PCB in a way other than its designers intended. However, the resistor value is set at the time of manufacture, removing one of the key methods of setting the LED's intensity.
Alphanumeric LEDs use the same drive strategy as indicator LEDs, the only difference being the larger number of channels, each with its own resistor. Seven-segment and starburst LED arrays are available in both common-anode or common-cathode form.
Finally, LEDs can be run from a single cell by use of a constant current switched mode invertor. The extra expense makes this option unpopular.
Lighting LEDs on mains
LEDs by their very nature, require constant current with low voltage, as oppose to the electrical grid which supplies high voltage with AC.
A CR dropper followed by full wave rectification is the usual ballast with series-parallel LED clusters. A single series string minimises dropper losses, while paralleled strings increase reliability. In practice usually three strings or more are used.
Operation on square wave and modified sine wave (MSW) sources, such as many invertors, causes heavily increased resistor dissipation in CR droppers, and LED ballasts designed for sine wave use tend to burn on non-sine waveforms. The non-sine waveform also causes high peak LED currents, heavily shortening LED life. An inductor & rectifier makes a more suitable ballast for such use, and other options are also possible.
Multiple LEDs can be connected in series with a single current limiting resistor provided the source voltage is greater than the sum of the individual LED threshold voltages. Parallel operation is also possible but can be more problematic. Parallel LEDs must have closely matched forward voltages (Vf) in order to have equal branch currents and, therefore, equal light output. Variations in the manufacturing process can make it difficult to obtain satisfactory operation when connecting some types of LEDs in parallel.[31]
To increase efficiency (or to allow intensity control without the complexity of a DAC), the power may be applied periodically or intermittently; so long as the flicker rate is greater than the human flicker fusion threshold, the LED will appear to be continuously lit.
Advantages of using LEDs
- Efficacy: LEDs produce more light per watt than incandescent bulbs; this is useful in battery powered or energy-saving devices.[32]
- Color: LEDs can emit light of an intended color without the use of color filters that traditional lighting methods require. This is more efficient and can lower initial costs.
- Size: LEDs can be very small (>2 mm2) and are easily populated onto printed circuit boards.
- On/Off time: LEDs light up very quickly. A typical red indicator LED will achieve full brightness in microseconds[33]. LEDs used in communications devices can have even faster response times.
- Cyceling: LEDs are ideal for use in applications that are subject to frequent on-off cycling, unlike fluorescent lamps that burn out more quickly when cycled frequently, or HID lamps that require a long time before restarting.
- Dimming: LEDs can very easily be dimmed either by Pulse-width modulation or lowering the forward current.
- Slow faliure: LEDs mostly fail by dimming over time, rather than the abrupt burn-out of incandescent bulbs.[34]
- Lifetime: LEDs can have a relatively long useful life. One report estimates 35,000 to 50,000 hours of useful life, though time to complete failure may be longer.[35] Fluorescent tubes typically are rated at about 30,000 hours, and incandescent light bulbs at 1,000–2,000 hours.[citation needed]
- Shock resistance:LEDs, being solid state components, are difficult to damage with external shock, unlike fluorescent and incandescent bulbs which are fragile.
- Focus: The solid package of the LED can be designed to focus its light. Incandescent and fluorescent sources often require an external reflector to collect light and direct it in a usable manner.
- Toxicity: LEDs do not contain mercury, unlike fluorescent lamps.
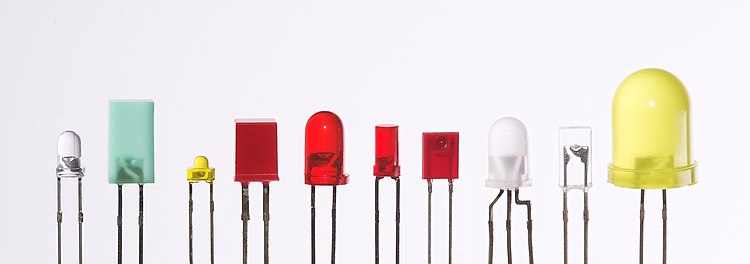
Disadvantages of using LEDs
- High price: LEDs are currently more expensive, price per lumen, on an initial capital cost basis, than more conventional lighting technologies. The additional expense partially stems from the relatively low lumen output and the drive circuitry and power supplies needed. However, when considering the total cost of ownership (including energy and maintenance costs), LEDs far surpass incandescent or halogen sources and begin to threaten compact fluorescent lamps[citation needed].
- Temperature dependence: LED performance largely depends on the ambient temperature of the operating environment. Over-driving the LED in high ambient temperatures may result in overheating of the LED package, eventually leading to device failure. Adequate heat-sinking is required to maintain long life. This is especially important when considering automotive, medical, and military applications where the device must operate over a large range of temperatures, and is required to have a low failure rate.
- Voltage sensitivity: LEDs must be supplied with the voltage above the threshold and a current below the rating. This can involve series resistors or current-regulated power supplies.[36]
- Light quality: Most white LEDs have spectra that differ significantly from a black body radiator like the sun or an incandescent light. The spike at 460 nm and dip at 500 nm can cause the color of objects to be perceived differently under LED illumination than sunlight or incandescent sources, due to metamerism,[37] red surfaces being rendered particularly badly by typical phosphor based LEDs white LEDs. However, the color rendering properties of common fluorescent lamps are often inferior to what is now available in state-of-art white LEDs.
- Area light source: LEDs do not approximate a “point source” of light, so cannot be used in applications needing a spherical light field. LEDs are not capable of providing divergence below a few degrees. This is contrasted with lasers, which can produce beams with divergences of 0.2 degrees or less.[38]
- Blue Hazzard: There is increasing concern that blue LEDs and white LEDs are now capable of exceeding safe limits of the so-called blue-light hazard as defined in eye safety specifications such as ANSI/IESNA RP-27.1-05: Recommended Practice for Photobiological Safety for Lamp and Lamp Systems.[39][40]
- Blue pollution: Because white LEDs emit much more blue light than conventional outdoor light sources such as high-pressure sodium lamps, the strong wavelength dependence of Rayleigh scattering means that LEDs can cause more light pollution than other light sources. It is therefore very important that LEDs are fully shielded when used outdoors. Compared to low-pressure sodium lamps, which emit at 589.3nm, the 460 nm emission spike of white and blue LEDs is scattered about 2.7 times more by the Earth's atmosphere. LEDs should not be used for outdoor lighting near astronomical observatories.
Types
The main types of LEDs are miniature, high power devices and custom designs such as alphanumeric or multi-color.
Miniature LEDs

These are mostly single-die LEDs used as indicators, and they come in various-size packages:
- surface mount
- 2 mm
- 3 mm (T1)
- 5 mm (T1³⁄₄)
- 10 mm
- Other sizes are also available, but less common.
Common package shapes:
- Round, dome top
- Round, flat top
- Rectangular, flat top (often seen in LED bar-graph displays)
- Triangular or square, flat top
The encapsulation may also be clear or semi opaque to improve contrast and viewing angle.
There are three main categories of miniature single die LEDs:
- Low current — typically rated for 2 mA at around 2 V (approximately 4 mW consumption).
- Standard — 20 mA LEDs at around 2 V (approximately 40 mW) for red, orange, yellow & green, and 20 mA at 4–5 V (approximately 100 mW) for blue, violet and white.
- Ultra-high output — 20 mA at approximately 2 V or 4–5 V, designed for viewing in direct sunlight.
Five- and twelve-volt LEDs
These are ordinary miniature LEDs that incorporate a suitable series resistor for direct connection to a 5 V or 12 V supply.
Flashing LEDs
Flashing LEDs are used as attention seeking indicators where it is desired to avoid the complexity of external electronics. Flashing LEDs resemble standard LEDs but they contain an integrated multivibrator circuit inside which causes the LED to flash with a typical period of one second. In diffused lens LEDs this is visible as a small black dot. Most flashing LEDs emit light of a single color, but more sophisticated devices can flash between multiple colors and even fade through a color sequence using RGB color mixing.
High power LEDs

High power LEDs (HPLED) can be driven at more than one ampere of current and give out large amounts of light. Since overheating is destructive, the HPLEDs must be highly efficient to minimize excess heat; furthermore, they are often mounted on a heat sink to allow for heat dissipation. If the heat from a HPLED is not removed, the device will burn out in seconds.
A single HPLED can often replace an incandescent bulb in a flashlight, or be set in an array to form a powerful LED lamp.
LEDs have been developed that can run directly from mains power without the need for a DC converter. For each half cycle part of the LED diode emits light and part is dark, and this is reversed during the next half cycle. Current efficiency is 80 lm/W.[41]
Multi-color LEDs
A “bi-color LED” is actually two different LEDs in one case. It consists of two dies connected to the same two leads but in opposite directions. Current flow in one direction produces one color, and current in the opposite direction produces the other color. Alternating the two colors with sufficient frequency causes the appearance of a blended third color. For example, a red/green LED operated in this fashion will color blend to produce a yellow appearance.
A “tri-color LED” is also two LEDs in one case, but the two LEDs are connected to separate leads so that the two LEDs can be controlled independently and lit simultaneously. A three-lead arrangement is typical with one commmon lead (anode or cathode).
RGB LEDs contain red, green and blue emitters, generally using a four-wire connection with one common lead (anode or cathode).
The Taiwanese LED manufacturer Everlight has introduced a 3 watt RGB package capable of driving each die at 1 watt.
Alphanumeric LEDs

LED displays are available in seven-segment and starburst format. Seven-segment displays handle all numbers and a limited set of letters. Starburst displays can display all letters.
Seven-segment LED displays were in widespread use in the 1970s and 1980s, but increasing use of liquid crystal displays, with their lower power consumption and greater display flexibility, has reduced the popularity of numeric and alphanumeric LED displays.
LED applications
List of LED applications
The many application of LEDs are very diverse but fall into three major categories: Visual signal application where the light goes more or less directly from the LED to the human eye, to convey a message or meaning. Illumination where LED light is reflected from object to give visual response of these objects. And finally LEDs are also used to generate light for measuring and interacting with processes that do not involve the human visual system.
Indicators and signs



- Status indicators on a variety of equipment
- LED Panels used as stadium displays, large television displays, electronic billboards and dynamic decorative displays.
- Traffic lights and signals
- Exit signs
- Thin, lightweight message displays at airports and railway stations, and as destination displays for trains, buses, trams, and ferries.
- Red or yellow LEDs are used in indicator and alphanumeric displays in environments where night vision must be retained: aircraft cockpits, submarine and ship bridges, astronomy observatories, and in the field, e.g. night time animal watching and military field use.
- Red, yellow, green, and blue LEDs can be used for model railroading applications
- In dot matrix arrangements for displaying messages.
- Because of their long life and fast switching times, LEDs have been used for automotive high-mounted brake lights and truck and bus brake lights and turn signals for some time, but many high-end vehicles are now starting to use LEDs for their entire rear light clusters. Besides the gain in reliability, this has styling advantages because LEDs are capable of forming much thinner lights than incandescent lamps with parabolic reflectors. The significant improvement in the time taken to light up (perhaps 0.5s faster than an incandescent bulb) improves safety by giving drivers more time to react. It has been reported that at normal highway speeds this equals one car length increased reaction time for the car behind. White LED headlamps are beginning to make an appearance.
- As a medium quality voltage reference in electronic circuits. The forward voltage drop (e.g., about 1.7 V for a normal red LED) can be used instead of a Zener diode in low-voltage regulators. Although LED forward voltage is much more current-dependent than a good Zener, Zener diodes are not available below voltages of about 3 V.
- Glowlights, as a more expensive but longer lasting and reusable alternative to Glowsticks.
- Lumalive, a photonic textile
- Emergency vehicle lighting
- LED-based Christmas lights available in different colors and with low energy consumption.
- LED-modules provide LEDs in a more usable form to people with less knowledge of electronics and soldering: the actual LEDs are contained within in protective and mountable casing, and a lead enables connection to power supply, typically 12 volts. LED modules are available in a wide range of shapes, sizes and colors.
Lighting


- Replacement light bulbs
- Flashlights with low energy usage and high durability
- Lanterns
- Streetlights
- Large scale video displays
- Architectural lighting
- Light source for machine vision systems, requiring bright, focused, homogeneous and possibly strobed illumination.
- Vehicle lighting on cars, motorcycles and bicycle lights
- Backlighting for LCD televisions and lightweight laptop displays. Using RGB LEDs increase the color gamut by as much as 45%.
- Light source for DLP projectors
- Stage lights using banks of RGB LEDs to easily change color and decrease heating from traditional stage lighting.
- Medical lighting where IR-radiation and high temperatures are unwanted.
- Strobe lights or camera flashes that operate at a safe, low voltage, as opposed to the 250+ volts commonly found in xenon flashlamp-based lighting. This is particularly applicable to cameras on mobile phones, where space is at a premium an bulky voltage-increasing circuitry is undesirable.
Non visual applications

- Grow lights using LEDs to increase photosynthesis in plants[42]
- Remote controls, such as for TVs and VCRs, often use infrared LEDs.
- Movement sensors, for example in optical computer mice. The Nintendo Wii's sensor bar uses infrared LEDs.
- In optical fiber and Free Space Optics communications.
- In pulse oximeters for measuring oxygen saturation
- LED phototherapy for acne using blue or red LEDs has been proven to significantly reduce acne over a three-month period.[citation needed]
- Some flatbed scanners use arrays of RGB LEDs rather than the typical cold-cathode fluorescent lamp as the light source. Having independent control of three illuminated colors allows the scanner to calibrate itself for more accurate color balance, and there is no need for warm-up. Furthermore, its sensors only need be monochromatic, since at any one point in time the page being scanned is only lit by a single color of light.
- Sterilization of water and other substances using UV light.[4]
- Touch sensing: Since LEDs can also be used as photodiodes, they can be used for both photo emission and detection. This could be used in for example a touch-sensing screen that register reflected light from a finger or stylus[43].
- Opto-isolators use an LED combined with a photodiode or phototransistor to provide a signal path with electrical isolation between two circuits. This is especially useful in medical equipment where the signals from a low voltage sensor circuit (usually battery powered) in contact with a living organism must be electrically isolated from any possible electrical failure in a recording or monitoring device operating at potentially dangerous voltages. An optoisolator also allows information to be transferred between circuits not sharing a common ground potential.
Light sources for machine vision systems
Machine vision systems often require bright and homogeneous illumination, so features of interest are easier to process. LEDs are often used to this purpose, and this field of application is likely to remain one of the major application areas until price drops low enough to make signaling and illumination applications more widespread. LEDs constitute a nearly ideal light source for machine vision systems for several main reasons:
- Size of illuminated field is usually comparatively small and Vision systems or smart camera are quite expensive, so cost of LEDs is usually a minor concern, compared to signaling applications.
- LED elements tend to be small and can be placed with high density over flat or even shaped substrates (PCBs etc) so that bright and homogeneous sources can be designed which direct light from tightly controlled directions on inspected parts.
- LEDs often have or can be used with small, inexpensive lenses and diffusers, helping to achieve high light densities and very good lighting control and homogeneity.
- LEDs can be easily strobed (in the microsecond range and below) and synchronized; their power also has reached high enough levels that sufficiently high intensity can be obtained, allowing well lit images even with very short light pulses: this is often used in order to obtain crisp and sharp “still” images of quickly-moving parts.
- LEDs come in several different colors and wavelengths, easily allowing to use the best color for each application, where different color may provide better visibility of features of interest. Having a precisely known spectrum allows tightly matched filters to be used to separate informative bandwidth or to reduce disturbing effect of ambient light.
- LEDs usually operate at comparatively low working temperatures, simplifying heat management and dissipation, therefore allowing plastic lenses, filters and diffusers to be used. Waterproof units can also easily be designed, allowing for use in harsh or wet environments (food, beverage, oil industries).
- LED sources can be shaped in several main configurations (spot lights for reflective illumination; ring lights for coaxial illumination; back lights for contour illumination; linear assemblies; flat, large format panels; dome sources for diffused, omnidirectional illumination).
- Very compact designs are possible, allowing for small LED illuminators to be integrated within smart cameras and vision sensors.
See also
- Nystagmus An eye condition in which sufferers have difficulty focusing on LED displays
- Photometry (optics) Main Photometry/Radiometry article—explains technical terms
- LED lamp—solid state lighting (SSL)
- Flashlight about the growing use of LED technology in Flashlights
- Blinkies
- Throwies
- LED circuit
- Nixie tube
- Light Up the World Foundation
- Lumalive, a photonic textile
References
- Cited
- ^ "LED". Retrieved 2008-01-04.
- ^ ... (2008). "Modeling the radiation pattern of LEDs". ... Optics Express. Retrieved 2008-01-25.
{{cite journal}}:|author=has numeric name (help) - ^ I. Moreno (2006). "LED Intensity Distribution" (PDF). International Optical Design Conference. International Optical Design, Technical Digest. Retrieved 2007-08-13.
- ^ a b c Development of a new water sterilization device with a 365 nm UV-LED, Medical and Biological Engineering and Computing, Volume 45, Number 12 / December, 2007
- ^ Tennessen, D.J. and Singsaas, E.L. and Sharkey, T.D. (1994). "Light-emitting diodes as a light source for photosynthesis research". Photosynthesis Research. Springer: 85–92.
{{cite journal}}:|access-date=requires|url=(help)CS1 maint: multiple names: authors list (link) - ^ H. J. Round (1907). "A Note on Carborundum". Electrical World. 19: 309.
- ^ Zheludev, N. (2007). "The life and times of the LED — a 100-year history" (PDF). Nature Photonics. 1 (4): 189–192. doi:10.1038/nphoton.2007.34.
- ^ Margolin J. "The Road to the Transistor".
- ^ Braunstein, Rubin (1955.). ""Radiative Transitions in Semiconductors,"". Physical Review. 99: 1892-3. doi:10.1103/PhysRev.99.1892.
{{cite journal}}: Check date values in:|year=(help) - ^ "The first LEDs were infrared (invisible)". The Quartz Watch. The Lemelson Center. Retrieved 2007-08-13.
- ^ "Nick Holonyak, Jr. 2004 Lemelson-MIT Prize Winner". Lemenson-MIT Program. Retrieved 2007-08-13.
- ^ Wolinsky, Howard (February 5 2005). "U. of I.'s Holonyak out to take some of Edison's luster". Chicago Sun-Times. Retrieved 2007-07-29.
{{cite news}}: Check date values in:|date=(help); Italic or bold markup not allowed in:|publisher=(help) - ^ "Brief Biography – Holonyak, Craford, Dupuis" (PDF). Technology Administration. Retrieved 2007-05-30.
- ^ "2006 Millennium technolgy prize awarded to UCSB's Shuji Nakamura". Retrieved 2007-05-30.
- ^ Cho, H.K., Jang, J., Choi, J.H., Choi, J., Kim, J., Lee, J.S., Lee, B., Choe, Y.H., Lee, K.D., Kim, S.H.; et al. (2006,). "Light extraction enhancement from nano-imprinted photonic crystal GaN-based blue light-emitting diodes,". Optics Express,. 14, (19). OSA: 8654--8660.
{{cite journal}}: Check date values in:|year=(help); Explicit use of et al. in:|author=(help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ "New efficient bulb sees the light" (Web). A new type of super-efficient household light bulb is being developed which could spell the end of regular bulbs. BBC News. Friday, 28 December 2007. Retrieved 2008-01-01.
{{cite web}}: Check date values in:|date=(help) - ^ "LEDs move into the ultraviolet". physicsworld.com. May 17 2006. Retrieved 2007-08-13.
{{cite news}}: Check date values in:|date=(help) - ^ "Alumni society honors four leaders in engineering and technology". Berkeley Engineering News. 2000-09-04. Retrieved 2007-01-23.
{{cite web}}: Check date values in:|date=(help) - ^ "GaN-based blue light emitting device development by Akasaki and Amano" (pdf). Takeda Award 2002 Achievement Facts Sheet. The Takeda Foundation. 2002-04-05. Retrieved 2007-11-28.
{{cite web}}: Check date values in:|date=(help) - ^ "United States Patent No. 5,578,839 (Nakamura et al.)". United States Patent and Trademark Office. filed 1993-11-17. Retrieved 2007-01-23.
{{cite web}}: Check date values in:|date=(help) - ^ Sensor Electronic Technology, Inc.: Nitride Products Manufacturer
- ^ "Jan Henrik Wold and Arne Valberg". 2001.
{{cite news}}: Unknown parameter|publish=ignored (help) - ^ ... (2007). "Color distribution from multicolor LED arrays". ... Optics Express. Retrieved 2008-09-10.
{{cite journal}}:|author=has numeric name (help) - ^ "E. Fred Schubert and Jong Kyu Kim". 2005.
{{cite news}}: Unknown parameter|publish=ignored (help) - ^ "Seoul Semiconductor squeezes 240 lumens into "brightest" LED". engadget. December 12 2006. Retrieved 2007-08-13.
{{cite news}}: Check date values in:|date=(help) - ^ "Nichia Unveils White LED with 150 lm/W Luminous Efficiency". Tech-On!. December 21 2006. Retrieved 2007-08-13.
{{cite news}}: Check date values in:|date=(help) - ^ Datasheet is not sufficient to confirm the claim, comparing Philips LXHL-LW6C and OSRAM LUW W5AM-LXLY-6P7R
- ^ "Candlepower Pink LED Reviews". Retrieved 2008-09-19.
- ^ "Accidental Invention Points to End of Light Bulbs". LiveScience.com. October 21 2005. Retrieved 2007-01-24.
{{cite news}}: Check date values in:|date=(help) - ^ Quantum-dot LED may be screen of choice for future electronics Massachusetts Institute of Technology News Office,December 18, 2002
- ^ "Electrical properties of GaN LEDs & Parallel connections" (PDF). Application Note. Nichia. Retrieved 2007-08-13.
- ^ "Solid-State Lighting: Comparing LEDs to Traditional Light Sources".
- ^ Philips Lumileds technical datasheet DS23 for the Luxeon Star states "less than 100ns".
- ^ "Solid-State Lighting: Lumen Depreciation".
- ^ http://www.netl.doe.gov/ssl/PDFs/lifetimeWhiteLEDs_aug16_r1.pdf
- ^ The Led Museum
- ^ James A. Worthey. "How White Light Works". LRO Lighting Research Symposium, Light and Color. Retrieved 2007-10-06.
- ^ Hecht, E: "Optics", Fourth Edition, page 591. Addison Wesley, 2002.
- ^ "Blue LEDs: A health hazard?". texyt.com. January 15 2007. Retrieved 2007-09-03.
{{cite news}}: Check date values in:|date=(help) - ^ "Light Impacts: Science News Online, [[May 27]], [[2006]]".
{{cite web}}: URL–wikilink conflict (help) 071214 sciencenews.org - ^ "Seoul Semiconductor launches AC LED lighting source Acriche". LEDS Magazine. Retrieved 2008-02-17.
- ^ Goins, GD and Yorio, NC and Sanwo, MM and Brown, CS (1997). "Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting". Journal of Experimental Botany. 48 (7). Soc Experiment Biol: 1407. doi:10.1093/jxb/48.7.1407.
{{cite journal}}: Unknown parameter|keywords=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dietz, Yerazunis, and Leigh (2004). "Very Low-Cost Sensing and Communication Using Bidirectional LEDs".
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link)
- General
- Shuji Nakamura, Gerhard Fasol, Stephen J Pearton The Blue Laser Diode: The Complete Story, Springer Verlag, 2nd Edition (October 2, 2000)
- Mills, Evan (2005). "The Specter of Fuel-Based Lighting". Science. 308: 1263–1264. doi:10.1126/science.1113090. PMID 15919979.
- Moreno, I., "Spatial distribution of LED radiation," in The International Optical Design Conference, Proc. SPIE vol. 6342, 634216:1-7 (2006).
- Salisbury, David F. (October 20, 2005). "Quantum dots that produce white light could be the light bulb's successor". Exploration—The Online Research Journal of Vanderbilt University.
{{cite web}}: Check date values in:|date=(help) (More details regarding the use of quantum dots as a phosphor for white LEDs.)
External links
- Solid State Lighting program at U.S. DOE
- Photonics Sources Group, Tyndall National Institute GaN and other photonics research at the Tyndall National Institute, Ireland.
- Solid State Lighting, Michael Shur - Rensselaer Polytechnic
- Applications notes about Discrete LEDs including basic driver circuits
- LED Circuitry Tutorial
- LED Resistor Calculator



