light emitting diode
A light emitting diode (short LED of English light-emitting diode , dt., Light emitting diode 'also luminescent diode ) is a semiconductor -Bauelement, the light radiates, if electric current in the forward direction flows. The LED blocks in the opposite direction. The electrical properties of the LED thus correspond to those of a diode . The light can be visible to the human eye or in the range of infrared radiation or ultraviolet radiation . The wavelength depends on the semiconductor material and the doping .
In the first three decades since it entered the market in 1962, LEDs were initially used as a light indicator and for signal transmission. Thanks to technological improvements, the luminous efficacy increased and the end of the 1990s was followed by applications in the field of LED light sources in everyday use.

The LED as a semiconductor
construction

The semiconductor crystal of many light-emitting diodes is soldered to the bottom of a conical recess in a metal holder. The inside of the recess acts as a reflector for the light emerging from the sides of the crystal. The soldering point forms one of the two electrical connections of the crystal. At the same time, it absorbs the waste heat that arises because the semiconductor crystal only converts part of the electrical power into light. In the case of wired light-emitting diodes, the holder with the reflector is designed as a wire with a rectangular cross-section, which serves as an electrical connection. Unlike other electronic components, the connecting wire is not made of tinned copper, but tinned steel. The thermal conductivity of steel is comparatively low. As a result, the semiconductor crystal is not destroyed by overheating when the component is soldered into a circuit board.
The top of the crystal is only electrically connected to the second steel connection wire by a thin bonding wire , so that the connection only covers very little of the light-emitting surface.
The cathode (-) is marked by a flat on the collar of the housing base. In the case of brand-new light-emitting diodes, the connection of the cathode is also shorter. Watch rule: K athode = k URZ = K ante. In most light-emitting diodes, the reflector is the cathode. In rare cases the structure is reversed. With regard to the circuit symbol (see below), the rule of thumb is that the technical direction of the current is "indicated" by the arrow that the anode (+) forms due to its shape.
High-performance light-emitting diodes (H-LED) are operated with currents higher than 20 milliamps. There are special requirements for heat dissipation, which are expressed in special designs. The heat can be dissipated through the power supply lines, the reflector trough or through heat conductors, which are heat conductors incorporated into the light-emitting diode body. Most H-LEDs from 1 watt upwards are prepared for mounting on heat sinks . In the case of LEDs (unlike incandescent lamps), a high temperature leads to a reduction in efficiency , and the expected service life is also shortened.
Another possibility is the direct wire bonding of the LED chip on the board ( chip on board ) and the later potting with silicone. These lamps are called "COB-LED" in specialist shops.
Multi-colored light-emitting diodes consist of several (2… 4) diodes in one housing. Most often they have a common anode or cathode and a connector for each color. In an embodiment with two connections, two light-emitting diode chips are connected in anti-parallel . Depending on the polarity, one or the other diode lights up. A quasi-stepless change in color can be achieved using a variable pulse width ratio of a suitable alternating current.
Working principle
The basic structure of a light-emitting diode corresponds to that of a pn semiconductor diode ; Light-emitting diodes therefore have the same basic properties. There is a big difference in the semiconductor material used. While non-luminous diodes are made from silicon , more rarely from germanium or selenium , the starting material for light-emitting diodes is a direct semiconductor , usually a gallium compound as a III-V compound semiconductor .
If a forward voltage is applied to a semiconductor diode, electrons migrate from the n-doped side to the pn junction. After the transition to the p-doped side, the electron then moves into the energetically more favorable valence band . This transition is called recombination , because it can also be interpreted as the meeting of an electron in the conduction band with a defect electron (hole). The energy released during recombination is usually emitted directly as light (photon) in a direct semiconductor.
In addition to the direct radiating recombination, the participation of excitons and phonons is also possible, which leads to slightly less energetic radiation (the color of the emitted light is shifted to reddish). This mechanism plays a role especially in the case of excitonic emission in green gallium phosphide light-emitting diodes.
Special variants that are not directly counted as light emitting diodes but are based on similar operating principles are the laser diode , the resonant cavity light emitting diode (RCLED or RC LED) and the organic light emitting diode (OLED).
Choice of material - indirect and direct semiconductors

left: direct semiconductor (e.g. gallium arsenide ),
right: indirect semiconductor (e.g. silicon)
The band structure of the semiconductor determines, among other things, the behavior of the energy transfer when an electron passes from the conduction band to the valence band and vice versa. The graphic on the right shows two simplified band structure diagrams. The course of the conduction and valence bands is plotted over the wave vector , clearly comparable to a reciprocal spatial coordinate that characterizes the necessary momentum transfer. The two basic forms of semiconductors or band transitions are shown: on the left a radiant transition of a direct semiconductor and on the right a transition of an indirect semiconductor.
In the case of indirect semiconductors such as silicon, the change of electrons from the conduction band minimum to the valence band maximum requires an additional pulse transfer in order to guarantee the conservation of the pulse . The impulse transfer takes place z. B. by the emission or absorption of a phonon ( lattice vibration ). The condition that an additional quasiparticle must be involved in the transition reduces its probability. Indirect semiconductors are therefore not suitable as light emitting diodes. Non-radiating transitions dominate, such as recombination via defects (Shockley-Read-Hall recombination). Correspondingly, a normal rectifier diode , for example, does not light up .
In contrast to this are the direct semiconductors , they are characterized by a “direct band transition”, which means that the electrons at the lower edge of the conduction band (conduction band minimum) and at the upper end of the valence band (valence band maximum) have the same momentum. This enables a direct transition of the electron with the emission of a photon (light), without the need for a phonon to maintain momentum. The quantum yield of the direct semiconductor gallium arsenide is around 0.5, with the indirect semiconductor silicon only around 1 · 10 −5 .
The energy of the emitted photon is equal to the energy of the band gap , i.e. the energetic distance between the conduction and valence bands.
as a numerical equation:
- λ ( W D ): wavelength of the emitted light. (For the numerical value equation in nm when inserted in eV .)
- h : Planck's quantum of action = 6.626 · 10 −34 Js = 4.13567 · 10 −15 eV s
- c : speed of light = 2.99792458 · 10 8 ms −1
- W D : work , here: band gap (given in eV for numerical value equations), depending on the semiconductor material used.
The size of the band gap, i.e. the energy gap , determines the energy, i.e. the frequency, wavelength or color of the emitted light. It can be controlled via the chemical composition of the semiconductor. The exchange of atoms in the crystal lattice changes the crystalline / molecular structure of the material. a. its lattice parameters or even its lattice structure . For example, the semiconductor gallium arsenide has a direct band gap of 1.4 eV, corresponding to a wavelength of 885 nm, that is, in the near infrared range . The addition of phosphorus increases the band gap, which makes the emitted light richer in energy, the wavelength decreasing and the color changing from infrared to red and yellow. However, the increase in phosphorus in the crystal also deforms the conduction band. If phosphorus replaces 50 percent of the arsenic atoms, the band gap is almost two electron volts, which corresponds to radiation of 650 nm (red), but the band structure has shifted so that direct radiation transitions are no longer observed, as in the example on the right shown. The energy of the band gap is also reflected in the level of the forward voltage of the diode. With long-wave light it is around 1.5 V, with blue light around 3 V, while silicon diodes have lower values of around 0.6 V.
Colors and technology
The color of a light-emitting diode depends essentially on the band gap of the semiconductor material used. The band gap can be varied within certain limits in the course of manufacture via the composition of the semiconductor. The color of a light emitting diode corresponds directly to a certain wavelength λ, i.e. H. the reciprocal of the frequency of the emitted electromagnetic radiation, multiplied by the speed of propagation. Examples of commonly used materials are:
- Aluminum gallium arsenide (AlGaAs) - infrared up to 1000 nm wavelength, red (665 nm)
- Gallium arsenide phosphide (GaAsP) and aluminum indium gallium phosphide (AlInGaP) - red, orange and yellow
- Gallium phosphide (GaP) - (yellow) green
- Indium Gallium Nitride (InGaN) / Gallium Nitride (GaN) - ultraviolet , violet, blue and green (true green)
White LEDs are mostly blue LEDs based on indium gallium nitride, with a yellowish luminescent layer in front of the LED chip , which acts as a wavelength converter and is primarily used in the field of LED lamps . LEDs have a similar structure, but with a modified luminescent layer, in pastel shades.
Seldom used materials for light emitting diodes include:
- Silicon carbide (SiC) - first commercial blue LED; hardly in practical use because of low efficiency
- Zinc Selenide (ZnSe) - blue emitter, but it never reached commercial maturity
| colour | Wavelength λ in nm | material |
|---|---|---|
| Infrared | λ> 760 |
Gallium Arsenide (GaAs) Aluminum Gallium Arsenide (AlGaAs) |
| 610 <λ <760 | Aluminum gallium arsenide (AlGaAs) gallium arsenide phosphide (GaAsP) aluminum gallium indium phosphide ( AlGaInP) gallium phosphide (GaP) |
|
| 590 <λ <610 | Gallium Arsenide Phosphide (GaAsP) Aluminum Gallium Indium Phosphide (AlGaInP) Gallium Phosphide (GaP) |
|
| 570 <λ <590 | Gallium Arsenide Phosphide (GaAsP) Aluminum Gallium Indium Phosphide (AlGaInP) Gallium Phosphide (GaP) |
|
| 500 <λ <570 |
Indium gallium nitride (InGaN) / gallium nitride (GaN) gallium phosphide (GaP) aluminum gallium indium phosphide ( AlGaInP) aluminum gallium phosphide (AlGaP) zinc oxide (ZnO), in development |
|
| 450 <λ <500 |
Zinc selenide (ZnSe) indium gallium nitride (InGaN) silicon carbide (SiC) silicon (Si) as a carrier, in development zinc oxide (ZnO), in development |
|
| 400 <λ <450 | Indium gallium nitride (InGaN) | |
| Ultraviolet | 230 <λ <400 |
Aluminum Nitride (AlN) Aluminum Gallium Nitride (AlGaN) Aluminum Gallium Indium Nitride (AlGaInN) Diamond (C) Experimental: Hexagonal Boron Nitride (BN) |
White LED
Since light-emitting diodes only generate monochromatic light , various methods of additive color mixing are used to generate white light.
Combination of different colored LEDs and phosphors
Red, green, blue light-emitting diodes, so-called RGB-LEDs, derived from the term RGB color space , are combined with one another in an LED housing in such a way that their light mixes well and thus appears white when the individual light-emitting diodes are appropriately controlled from the outside . Additional optical components such as a diffuser are usually required for better light mixing . With this combination of light-emitting diodes, light of different colors can also be produced by appropriate control of the individual light-emitting diodes, and smooth color transitions are also possible.
An ultraviolet LED (UV LED) is rarely combined with several different phosphors in red, green and blue, which allows good color rendering up to over R a = 90. Three relatively narrow peaks are generated in the spectrum, which stands for light in three narrow frequency bands. Different phosphor layer thicknesses, however, lead to an inhomogeneous light color, which is dependent on the direction of emission, particularly at the edge; nor can the color temperature or color be changed during operation as with RGB LEDs.
Luminescence
A blue or ultraviolet LED is combined with a photoluminescent dye, also known as a phosphor . Similar to fluorescent tubes , short-wave, higher-energy light such as the blue light of LEDs can be converted into long-wave light. The choice of phosphors can vary and determines the color temperature.
In the most common manufacturing process for white LEDs, gallium nitride is applied epitaxially , usually by means of organometallic gas phase epitaxy (MOVPE), to a carrier (substrate) made of sapphire. This creates the first layer of the GaN semiconductor crystal. The light-emitting layer usually consists of InGaN, the blue light of which is partially converted into longer-wave light by the phosphor. In a new process, the essential principles of which were developed in 2000 at the Otto von Guericke University Magdeburg , the expensive sapphire substrate is replaced by silicon. After a first AlN layer, the gallium nitride is then grown on the silicon. Such LEDs are only efficient, however, if the light-absorbing silicon substrate is removed and replaced by a highly reflective layer, usually based on silver , as is now the case for high-power LEDs on sapphire substrates. With this method, the much cheaper and large-area silicon wafers can be used for LED production, and the process of removing it from the substrate is greatly simplified.
The type of fluorescent coating is decisive for the quality. As you can see from the graphic, the luminescent layer, which is yellowish on average, generates a very broadband light, which leads to a balanced spectrum. On the other hand, the overlap with the spectra of most red dyes is poor, which impairs the color rendering and, for example, in color LCDs that are backlit with such white LEDs , leads to poor red rendering.
properties
Spectral characteristics
In contrast to incandescent lamps , light-emitting diodes are not heat emitters . Colorful (non-white) light-emitting diodes emit light in a limited spectral range; the light is almost monochromatic . This is why they are particularly efficient when used as signal light compared to other light sources in which color filters have to absorb the largest part of the spectrum in order to achieve a monochrome color characteristic. For the use of light-emitting diodes for general lighting purposes, blue light-emitting diodes are usually combined with phosphors. In addition to the broad spectrum of the fluorescent substance, they have a narrower blue light component.
Until the beginning of the 1990s, light-emitting diodes could not be produced in sufficient quality for all colors of the visible spectrum; blue light-emitting diodes in particular were not available. The use of green light-emitting diodes was also not possible for traffic lights because of the required blue-green light color. The development of the first blue-green light-emitting diodes goes back to the work of Isamu Akasaki in 1989 on the basis of the material gallium nitride . The mass production of blue-green and then blue light-emitting diodes began in 1993.
Electrical Properties
Light-emitting diodes have an exponentially increasing current-voltage characteristic , which among other things also depends on the temperature. The luminous flux is almost proportional to the operating current.
The forward voltage or the voltage across the diode is set by the operating current, has specimen variance and is temperature-dependent - it decreases with increasing temperature, as with all semiconductor diodes. The supply via a constant current source (often approximated in the form of a series resistor) is therefore important for a defined luminosity. Direct operation on a voltage source is very risky, since the operating point cannot be set with sufficient accuracy via the voltage for the desired current due to the specimen variance and the temperature dependency.
In the course of development, the light output was increased by optimizing the semiconductor material and the geometry of the semiconductor crystal and housing. From around the 1990s, this made it possible to operate LEDs with a very low current ( low-current LEDs) and still achieve a reasonable level of brightness. The maximum permissible current consumption of LEDs ranges from 2 mA (for example with miniaturized SMD LEDs or low-current LEDs) to 20 mA (standard LEDs) to over 18 A (as of June 2008) for high-performance LEDs. The forward voltage U f (for English forward voltage ) depends on the semiconductor material, which in turn determines the color of light. Indicators for the voltage drop are:
- Infrared LED: 1.2-1.8 V, typ. 1.3 V
- Red: 1.6-2.2V
- Yellow, green: 1.9-2.5V
- Blue, white: 2.7-3.5V
- UV-LED: 3.1-4.5 V, typ. 3.7 V.
The forward voltage depends u. a. depends on the current density in the LED and on series resistances (rail resistance in the crystal) in the diode and the contacts, bonding wires and connections and, as a result, increases with more powerful LEDs.
The maximum permissible reverse voltage is usually 5 volts, above which the LED is usually destroyed.
Optical properties
Light emitting diodes are usually encapsulated with polymers . Glass, ceramic or metal housings are also used for bright LEDs. Metal housings, mostly made of aluminum , serve to dissipate heat. The plastic body is often shaped like a lens and lies over the crystal. It reduces the critical angle of total reflection and bundles the emitted radiation power into a smaller, definable solid angle.
An important parameter of an LED is the opening angle .
| Opening angle | 180 ° | 170 ° | 160 ° | 150 ° | 140 ° | 130 ° | 120 ° | 110 ° | 100 ° | 95 ° |
|---|---|---|---|---|---|---|---|---|---|---|
| sr factor | 6.2832 | 5.7356 | 5.1921 | 4.6570 | 4.1342 | 3.6278 | 3.1416 | 2.6793 | 2.2444 | 2.0383 |
| Opening angle | 90 ° | 85 ° | 80 ° | 75 ° | 70 ° | 65.55 ° | 60 ° | 55 ° | 50 ° | 45 ° |
| sr factor | 1.8403 | 1.6507 | 1.4700 | 1.2984 | 1.1363 | 1,0003 | 0.8418 | 0.7099 | 0.5887 | 0.4783 |
| Opening angle | 40 ° | 35 ° | 30 ° | 25 ° | 20 ° | 15 ° | 10 ° | 5 ° | 1 ° | |
| sr factor | 0.3789 | 0.2908 | 0.2141 | 0.1489 | 0.0955 | 0.0538 | 0.0239 | 0.0060 | 0.00024 |
Due to the limited opening angle, an LED, unlike an incandescent lamp, only illuminates a partial area (based on the surface of a sphere around the radiation source in the center). Several LEDs are required for 360 ° lighting with LEDs. To determine how many light-emitting diodes are required, the following equation derived from the spherical cap can be used.
describes the opening angle of the LED.
Example: To implement a 360 ° lamp with light-emitting diodes, z. B. 18 light-emitting diodes are necessary if they each have an opening angle of 55 ° (that is about an eighteenth of a sphere).
Light-emitting diodes with a lens that is designed to achieve rather large opening angles are also referred to as straw-hat LEDs. Occasionally, concave, conical shapes are used for this purpose; they work through total reflection, whereby light is reflected in such a way that it emerges from the housing laterally (approximately parallel to the emitter surface). The light emission of the LED chip or the fluorescent layer takes place in a half space. The lost heat is dissipated in the other direction.
Light intensity
The luminous intensity of LEDs is specified in the unit candela (cd) or parts thereof. In contrast to the luminous flux , the luminous intensity also takes into account the radiation characteristics in the form of the solid angle . Typical values are:
- 20 mcd to 50 mcd for classic LEDs for signal applications such as in control panels etc.
- up to 0.8 cd have newer so-called "super bright" LEDs, which are therefore also suitable for bright or daylight environments
- LED modules have up to 10 cd for lighting purposes, they are composed of several LED chips
Aging
Service life in switch-on hours
The service life (light degradation) of an LED is the time after which its luminous flux has fallen to an average of 70% of the initial value ( L70B50 value); some internet sources also speak of the end of life at 80% or 50% of the initial luminous flux.
The luminous flux of light-emitting diodes gradually decreases, but they usually do not fail suddenly. The aging is almost linear. The service life depends on the respective semiconductor material, the operating conditions (temperature, current) and the individually tolerable change in color temperature of the fluorescent dyes (white LEDs become more bluish). High temperatures (usually due to high currents) drastically shorten the life of the LEDs. With perfectly matched components, lifetimes of 50,000 hours and more can be achieved.
The aging of LEDs is primarily due to the enlargement of the imperfections in the crystal due to thermal influences. These areas no longer take part in the generation of light. Radiationless transitions arise. In the case of GaN LEDs in the blue and ultraviolet range, aging of the plastic housing due to the short-wave light with associated clouding can also be determined.
Applications and areas of use
In the 1970s and 1980s, the area of application of LEDs mainly comprised display elements such as status displays with the colors red, orange, yellow and yellow-green due to the low light output and the lack of availability of all colors. They replace smaller incandescent lamps or glow lamps . This area of application also includes displays such as seven-segment displays or matrix displays , in which they replaced special electron tubes such as the fluorescent display and its predecessor, the Nixie tube .
Since the beginning of the LED, there have also been applications for signal transmission where the function of the light-emitting diode is not necessarily optically visible to the user and where light-emitting diodes are still dominant today. Examples are infrared LEDs in infrared remote controls , in light barriers or in optocouplers for galvanic separation of electrical circuits.
With the market availability of high-performance and inexpensive blue (and therefore also white) light-emitting diodes since the 2000s, LEDs have established themselves in almost all lighting applications . In addition to room lighting or street lighting , LEDs are also used in flashlights , LED headlights as well as floodlight systems and effect lighting, e.g. B. in illuminated furniture, showcases, frames or even with items of clothing where narrow and partially hidden LED strips are intended to provide pleasant indirect lighting and room atmosphere. Today there is a wide variety of LED light sources , including LED filament lamps, which on the one hand imitate the appearance of traditional carbon filament light bulbs , while more powerful types resemble the appearance of incandescent lamps with tungsten filaments .
At the same time as the availability of high-performance light-emitting diodes for lighting purposes, there was also signaling with a higher light output, such as traffic lights, taillights and beacons on vehicles. In 2011, an airport apron was illuminated with LEDs for the first time in Europe: In Innsbruck, a system with 14 high masts illuminates an area of 49,000 m².
Another area of application of LEDs is the backlighting of liquid crystal screens , since LEDs have lighting that is more stable over the long term and, in some cases, require less electricity than cold cathode lamps . In addition, very small installation depths can be achieved in this way. Corresponding LCD televisions are often referred to colloquially as LED televisions .
In addition, there are special areas of application that make use of the spectral properties of the light-emitting diodes used. Examples are medical technology , where, among other things, ultraviolet LEDs are used to polymerize plastics in dental technology , red and infrared LEDs are used to measure oxygen saturation in pulse oximeters , or in light skin therapy - also known as LED photo rejuvenation .
LED development
history

Henry Joseph Round (1881–1966) first observed in 1907 that inorganic substances are capable of emitting light under the influence of an applied voltage. In 1921 the Russian physicist Oleg Lossew rediscovered the Round effect and examined it in more detail from 1927 to 1942, since he suspected that the phenomenon should be interpreted as a reversal of Einstein's photoelectric effect . Georges Destriau discovered a similar luminous phenomenon in zinc sulfide in 1935 and named it Lossew light after the Russian physicist .
In 1951, the development of the bipolar transistor made scientific progress in semiconductor physics possible. Since then it has been possible to elucidate the process of light emission. At first, experiments were continued with zinc sulfide; However, the research on III-V compound semiconductors recognized as semiconductors was more successful . From 1957 on, research into light generation concentrated entirely on semiconductors. The light emission in the visible range based on gallium arsenide (GaAs) and gallium phosphide (GaP) was particularly important.
Some sources attribute the invention of the light emitting diode to Nick Holonyak and date it to 1962.
In 1968, the American chemical company Monsanto was the first to mass-produce (red) LEDs based on gallium arsenide phosphide, and in the years that followed, the development continued. The serial production of the discrete LED and the seven-segment display made the first pocket calculators and digital wristwatches possible .
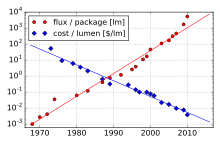
Since the first LEDs in 1962, the light output has increased from less than 0.1 lm / W to over 100 lm / W. These development steps , which are mostly made in leaps and bounds, are based on the ever better quality of the semiconductor layers (lower defect densities , less impurities) on the use of semiconductor heterostructures , low-dimensional structures ( quantum films , quantum dots ), transparent substrates and improved light extraction. Starting with GaAs / AlAs (1960s, red-yellow), new semiconductor materials such as GaP (1970s, green LEDs) and GaN (1980s / 1990s, green to UV) were developed, so that today, except for one Gap in the green-yellow area, LEDs in all colors of the spectrum are there.
Semiconductor materials that efficiently generate light in the short-wave range (blue, UV) have long been sought. The main problem was the doping of a p-conducting area of suitable wide-gap semiconductors, which was first achieved in 1988 with GaN by Isamu Akasaki's group in Japan, and then in 1992 by Shuji Nakamura with a different approach. This led to the first commercial blue LED based on GaN, which, in the meantime expanded to include white and green LEDs and blue laser diodes , has been marketed by Nichia since 1993 . Until then, blue LEDs were based on the material silicon carbide , which is poorly suited as an indirect semiconductor for efficient light emission. Isamu Akasaki, Hiroshi Amano and Shuji Nakamura received the 2014 Nobel Prize in Physics for “the invention of efficient blue light-emitting diodes that made bright and energy-saving white light sources possible”.
In 2006 a blue LED from Nichia achieved a light output of 150 lm / W in laboratory tests. This corresponds to the efficiency of sodium vapor lamps , which have been available in various designs since the 1970s. In 2007, the Cree company succeeded in operating a cold white LED with over 1000 lm and an efficiency of 72 lm / W in its laboratory; the warm white version still achieved a light output of 52 lm / W at 760 lm. An LED from Nichia has been on the market since 2009 with a specified luminous efficacy of 160 lm / W, but only with low output. Cree delivered the first LEDs in 2010, which reached 160 lm / W at 1 W and still approx. 100 lm / W at 10 W.
In September 2009, Cree began delivering a white LED with a luminous efficacy (manufacturer information) of 132 lm / W, which drops from almost 10 W to 105 lm / W at the maximum power consumption, with luminous flux values of 350 mA in the power classes for this type of production 114 lm; 122 lm; 130 lm and 139 lm (corresponds to 132 lm / W) are offered. In February 2010, the company reported on a laboratory prototype LED that reached 208 lm / W at room temperature, with a color temperature of 4579 K. In October 2011, Osram was able to present prototypes of a red LED that worked at 609 nm and a nominal current of 350 mA a light output of 168 lm / W is achieved.
When comparing the light intensity of different LEDs, the beam angle must be included in the calculation. Usual beam angles are between 24 ° and 40 °.
The focus of further development is primarily on increasing efficiency and further reducing production costs. In addition, work was carried out around 2015 in particular on producing transparent carrier and semiconductor materials as well as transparent electrical leads, since the bonding wires (electrical lines to the semiconductor chip) cover part of the luminous surface.
State of the art
Light output
The most efficient white LEDs achieve a luminous efficiency of 303 lm / W (as of March 2014, in September 2010 it was 250 lm / W). The theoretical maximum is approx. 350 lm / W when white light with 6600 K color temperature is generated with a physical efficiency of 100%.
Since the measurement in the lumen unit takes into account the properties of the human eye (see light sensitivity curve ), LEDs in the colors green to yellow achieve particularly high efficiency values, while blue LEDs, for example, perform significantly worse. In terms of purely physical efficiency, which indicates the conversion of electrical energy into light energy, blue LEDs are usually better. Physical efficiencies of up to around 85% can currently be achieved, based on the actual LED, without losses due to ballasts and possibly optics. In addition to such laboratory values, LEDs with 200 lm / W are in operation today.
Mass production
The efficiency of a mass-produced LED is subject to a certain spread. For example, individual LED laboratory samples with high efficiency were produced in the laboratory years ago and soon afterwards announced as a mass product. With the so-called "Fluxbinning" several classes of different luminous fluxes are selected from one production and each offered with different prices.
Operation and connection

With a constant semiconductor temperature, the luminous flux of a light-emitting diode is approximately proportional to the current flowing through it. The efficiency decreases with increasing temperature, u. a. therefore, the light yield drops at the performance limit depending on the type of cooling. Furthermore, the sheet resistance (semiconductor crystal, bonding wire) means that LEDs are less efficient at their specified rated power than with lower currents. The LED ages accelerated up to spontaneous failure if the temperature of the semiconductor exceeds approx. 150 ° C for a longer period of time.
Like other semiconductor diodes, a light-emitting diode has an exponential current-voltage characteristic . Small fluctuations in the voltage cause large changes in current from the threshold voltage.
The picture on the right shows a typical current-voltage characteristic of a white light-emitting diode, here with a nominal current of 350 mA. It can absorb this current under normal conditions without fear of overheating. A forward voltage of around 3.4 V can be read from its characteristic curve at nominal current, corresponding to a power consumption of around 1 W.
The red curve applies to an increased temperature (the band gap and forward voltage decrease with increasing temperature). The current can therefore increase by more than 50% even if the forward voltage is kept constant. An LED can therefore not be operated directly from a voltage source . LEDs can be connected to a current source , a constant current source or, by means of a measure to limit current, to a voltage source.
The voltage must be at least as high as the threshold voltage so that a significant current can flow. For example, a blue LED will remain dark when a voltage of 2.4 V is applied (two NiMh batteries , 1.2 V each in series). However, three such battery cells with a total of 3.6 V increase the power consumption to over 150%, the LED fails after a short time.
In pulsed operation for a few microseconds to a few milliseconds, LEDs can be operated with currents that are a multiple of the continuous rated current. In particular, infrared LEDs are specified for this. Their typical applications are infrared remote controls , in which LEDs are operated pulsed at around 40 kHz. The modulation of the light or radiation power is possible up to several 100 kHz to several 10 MHz, depending on the type of LED.
Operation with series resistor
The simplest way of supplying an LED to a voltage source is to connect a series resistor to it. In principle, the overall efficiency is no worse than that of a linearly regulated constant current source . If this arrangement is operated with a voltage source whose voltage U 0 is known under load (rated current I ), the desired current I can be set by selecting the resistor:
Example:
The power loss and thus the size of the resistor results from
The next higher normal value is 0.5 W.
In the case of an unregulated voltage source such as a power supply unit consisting of a transformer with rectifier and smoothing capacitor , the internal resistance of the source leads to a strong dependence of the output voltage on the load current. With the above formula it should be noted that U 0 is not the no-load voltage, but the output voltage at the nominal current I , which can be almost halved compared to the no-load voltage with small transformers (approx. 3 VA).
The disadvantage of a series resistor is that the current depends on the supply voltage. This is especially true when a relatively low voltage drops across the series resistor. It is therefore hardly possible to reduce the losses by only having a small voltage difference between the LED and the supply network (e.g. vehicle electrical system).
Example : Three 3.4 V LEDs are connected to a 12 V vehicle electrical system, so that with a voltage of U = 12 V, only 1.8 V remain for the series resistor. With a series resistor of 5.2 Ω, the result is a current of 348 mA. When charging the battery in the car, however, voltages of up to 14.4 V can occur. This would then result in a practically doubled current of around 700 mA (the voltage drop on the LED also increases slightly), although the on-board voltage has only increased by 16%. The selected version is therefore not reliable and therefore unsuitable. This could be remedied by reducing the number of light-emitting diodes connected in series or a constant current source instead of a series resistor.
Operation with constant current source
When operating light-emitting diodes on a constant current source, there is no problem of the current being dependent on the supply voltage. The LED can then be operated safely over a very wide voltage range. Constant current sources can be implemented with transistors or integrated circuits .
One possibility for realizing a constant current source is offered by a JFET in the form of a simple linear regulator that is connected to a voltage source in series with an LED. The adjacent circuit is connected in series to the LED instead of the series resistor R. By selecting R 1 , the current intensity through the LED can be adjusted. The resistance value depends on the parameters of the JFET and - in contrast to operation with a series resistor - not on the supply voltage. As a rough estimate, the constant current can be determined using the following equation:
( U GS is the voltage between gate and source; this value can be taken from the data sheet of the respective JFET and is equal to the voltage that is applied to resistor R 1 during operation .)
The typical supply voltage ranges that can be achieved with this circuit can span the range of a few volts up to 40 volts and are limited by the dielectric strength and maximum power loss of the JFET.
Operation with switching regulator
Linear circuits have the disadvantage that they convert the product of the voltage difference and the operating current in the form of power loss into heat. In the case of high-power LEDs with operating currents from a few 100 mA upwards, switching regulators ( buck converters ) are often used to minimize losses , which regulate to a constant output current. Switching regulators also offer the possibility of achieving the forward voltage of the LED (with white LEDs 2.5 V to over 4 V) even when operating on a single cell (battery, primary cell) ( step-up converter ).
Switching regulators often have efficiencies of more than 90% and therefore almost completely avoid the losses of the aforementioned solutions, even with high voltage differences. The high switching frequency and the fact that the current is kept constant ensure that LEDs operated in this way still glow largely flicker-free to the human eye. In addition, with electronics designed for this, the operating current and thus the brightness can be controlled using pulse width modulation (PWM). There are also special integrated circuits for this.
The simple circuit shown in the picture on the right, however, has no current control - the peak current that flows through the LED when the transistor is blocked is determined by the saturation current strength of the ferrite toroidal core coil and / or the current gain of the transistor.
Switching regulators have the disadvantage that the basic structure is more complex and they can cause interference , the suppression of which requires further effort.
Operation on mains voltage
An LED can be operated on mains voltage with a rectifier and a series capacitor. The sum of the forward voltages of the LEDs connected in series must be significantly lower than the mains voltage in order to keep the current sufficiently constant within the mains voltage tolerance. This solution, which is often used in lighting, also requires a resistor to limit the current and a filter capacitor that protects the rectifier or the LED from the inrush current caused by the series capacitor and from overvoltage pulses in the network. Almost flicker-free light can only be achieved with this method with losses and with a large filter capacitor. Dimmability with leading edge dimmers is not possible. The current consumption is not sinusoidal due to the non-linear LED characteristic.
In order to avoid the aforementioned disadvantages, relevant companies have developed a variety of LED drivers and driver IC with which u. a. the following properties can be achieved:
- constant brightness even with mains voltage and temperature fluctuations
- but can be dimmed with the usual leading edge or trailing edge dimmers, with flicker-free light
- Temperature management
- sinusoidal mains current consumption through power factor correction filter (PFC)
Many drivers offer analog or digital interfaces for brightness control. The circuit topologies are flyback converters , buck converters or resonance converters .
handling
LEDs not only have to be protected from excessively high forward current, but also from excessively high reverse voltage. Many manufacturers only give 3–5 V maximum reverse voltage. For this reason, LEDs must be protected from excessively high reverse voltage, especially when they are operated on AC voltage or when the polarity is reversed. This can be done by an anti-parallel diode (this can also be another LED). Like other semiconductor components, LEDs must therefore be protected from electrostatic discharge (ESD). They are delivered and stored in electrostatically dissipative packaging and may only be handled and processed with ESD protective measures. Some LEDs have built-in ESD protection.
literature
- M. George Craford : Visible Light-Emitting Diodes: Past, Present, and Very Bright Future . In: MRS Bulletin . tape 25 , no. 10 , 2000, pp. 27–31 , doi : 10.1557 / mrs2000.200 .
- Roland Heinz: Basics of light generation: from light bulbs to lasers, lamps, power reduction, LED, OLED, laser . 5th enlarged edition. Highlight, Rüthen 2015, ISBN 978-3-937873-05-3 .
- E. Fred Schubert : Light-Emitting Diodes . Cambridge University Press, Cambridge 2003, ISBN 0-521-53351-1 .
Web links
- Instructional video about the light emitting diode on YouTube (English)
- Light-emitting diode (basics and more)
Sources from manufacturers and suppliers
- ^ Research success : first gallium nitride LED chips on silicon in pilot status. OSRAM press release, January 12, 2012, accessed on January 12, 2012.
- ↑ Nichia Corporation (ed.): Specifications for white LED NF2W757GT-V1F1 (PDF; 516 kB). Retrieved August 20, 2020.
- ↑ Nicha Corporation (ed.): Specifications for Nichia Chip Type UV LED - Model: NCSU034B (PDF; 391 kB). Retrieved June 16, 2016.
- ↑ OSRAM, LED service life: long-lasting light quality , OSRAM, accessed on January 13, 2013.
- ↑ Phillips LED & OLED 2012 Principle 7 - The service life. ( Memento from January 31, 2012 in the Internet Archive ) (PDF; 1.3 MB). Retrieved January 13, 2013.
- ↑ Cree XP-G LED ( Memento of the original from March 13, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Cree Breaks 200 Lumens Per Watt Efficacy Barrier
- ↑ OSRAM trade press informs about laboratory record: red LED cracks 200 lm / W mark
- ↑ GU10 / GU5.3 spotlights - no lumen specification and only candela , ELVjournal
- ↑ 300lm / watt power LED
Individual evidence
- ↑ LED: Basics - Application - Effect . In: licht.de (Ed.): Licht.wissen . No. 17 . Frankfurt 2018, ISBN 978-3-945220-18-4 , pp. 29 .
- ↑ LEDs move into the ultraviolet , May 17, 2006.
- ^ Y. Kubota, K. Watanabe, O. Tsuda, T. Taniguchi: Deep Ultraviolet Light-Emitting Hexagonal Boron Nitride Synthesized at Atmospheric Pressure . In: Science . tape 317 , no. 5840 , 2007, p. 932-934 , doi : 10.1126 / science.1144216 , PMID 17702939 .
- ↑ Thomas Jüstel: Optimal phosphors for LED applications. (PDF) In: 9th conference: LED in lighting technology, Essen 12. – 13. March 2013. Münster University of Applied Sciences / Institute for Optical Technologies, accessed on February 14, 2018 .
- ↑ Tingkai Li, Michael Mastro, Armin Dadgar: III-V Compound Semiconductors: Integration With silicone-based Microelectronics . CRC Press, Boca Raton, FL 2010, ISBN 978-1-4398-1522-9 .
- ↑ Hiroshi Amano, Masahiro Kito, Kazumasa Hiramatsu, Isamu Akasaki: P-Type Conduction in Mg-Doped GaN Treated with Low-Energy Electron Beam Irradiation (LEEBI) . In: Japanese Journal of Applied Physics . tape 28 , 1989, pp. L2112-L2114 , doi : 10.1143 / JJAP.28.L2112 .
- ↑ Bart Van Zeghbroeck: Principles of Semiconductor Devices . 2004 (electronic copy here )
- ↑ LED: Basics - Application - Effect . In: licht.de (Ed.): Licht.wissen . No. 17 . Frankfurt 2018, ISBN 978-3-945220-18-4 , pp. 21 .
- ↑ [1] , accessed October 17, 2019
- ↑ The lighting with artificial light . In: licht.de (Ed.): Licht.wissen . 1st edition. Frankfurt 2016, ISBN 978-3-945220-03-0 , pp. 14 .
- ↑ LED: Basics - Application - Effect . In: licht.de (Ed.): Licht.wissen . No. 17 . Frankfurt 2018, ISBN 978-3-945220-18-4 , pp. 45 .
- ↑ LED light for the airport apron. news.ORF.at, published on October 19, 2011.
- ^ Henry Joseph Round: A note on carborundum . In: Elect World . tape 19 , 1907, pp. 309 .
- ↑ Nikolay Zheludev: The life and times of the LED - history a 100-year . In: Nature Photonics . tape 1 , no. 4 , p. 189–192 , doi : 10.1038 / nphoton.2007.34 ( PDF [accessed October 28, 2008]). PDF ( Memento of the original from March 31, 2017 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Ari Ben-Menahem: Historical Encyclopedia of Natural and Mathematical Sciences . Vol. 1. Springer Science & Business Media, 2009, ISBN 978-3-540-68831-0 , p. 3588 .
- ↑ The first light-emitting transistor. (No longer available online.) In: Spektrumdirekt. January 7, 2004, archived from the original ; Retrieved May 31, 2010 .
- ^ E. Fred Schubert : Light-Emitting Diodes . Cambridge University Press 2003, ISBN 0-8194-3956-8 , pp. 8-10 ( limited preview in Google Book Search).
- ^ Website of the Nobel Committee
- ↑ Press release from Nichia in LEDsMagazine.com
- ↑ http://www.ti.com/lit/ds/symlink/lm3447.pdf data sheet of the LM3447



























