Azacitidine
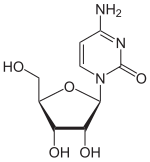
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Azacitidine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 12 N 4 O 5 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 244.21 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
228-230 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Azacitidine ( INN ), chemically 5-azacytidine (5-azaC) (trade name: Vidaza, manufacturer: Celgene ) is a synthetic nucleoside . It consists of β- D- ribofuranose (sugar) and a substituted triazine . It is a chemical analog of the nucleoside cytidine . The cytostatic substance is used as a drug in chemotherapy for malignant diseases of the blood-forming system. It prevents cancer cells from growing. 5- Azacytidine is isolated from Streptoverticillium ladakanus and also shows weak antibiotic activity.
properties
The 5- aza cytidine differs chemically from cytidine in that a CH unit in the 5-position is formally replaced by a nitrogen atom. The pyrimidine backbone thus becomes a 1,3,5-triazine backbone. The analogue with deoxyribose is decitabine .
|

|

|
| Cytidine, C. | 5-azacytidine, 5-azaC | Decitabine |
effect
When 5-azacytidine is present in the cell, it is incorporated into DNA during replication and into RNA during transcription . This incorporation of 5-azacytidine into DNA and RNA inhibits DNA methyltransferases or RNA methyltransferases and thereby causes demethylation in that sequence. This influences the proteins involved in cell regulation, which can bind to the DNA / RNA substrate.
Clinical information
Application areas (indications)
Azacitidine is indicated for the treatment of adult patients who are not suitable for a haematopoietic stem cell transplant ( stem cell transplantation ) and who have one of the following symptoms:
- myelodysplastic syndromes (MDS) with intermediate risk 2 or high risk according to the International Prognostic Scoring System (IPSS),
- chronic myelomonocytic leukemia (CMML) with 10–29% bone marrow blasts without myeloproliferative disorder,
- acute myeloid leukemia (AML) with 20–30% blasts and multiline dysplasia according to the classification of the World Health Organization (WHO).
Type and duration of application
Treatment with azacitidine should be started and monitored under the supervision of a doctor who has experience in the use of chemotherapy drugs. Patients should receive premedication with antiemetics for nausea and vomiting. Azacitidine is a powder that is made up into a suspension for injection.
Adverse effects (side effects)
The most commonly reported adverse reactions to azacitidine treatment were haematological reactions (71.4%) including thrombocytopenia , neutropenia and leukopenia (usually grade 3–4), gastrointestinal events (60.6%) including nausea, vomiting (in the Usually grade 1–2) and reactions at the injection site (77.1%; usually grade 1–2). In addition, anxiety, confusion, insomnia.
Other Information
Orphan drug
Azacitidine was granted orphan drug status by the Food and Drug Administration (FDA) back in 2004 . The term is used for drugs that are used to treat very rare diseases.
History (admission)
In 2004, Vidaza was approved for the US market by the Food and Drug Administration (FDA). The European Medicines Agency approved azacitidine for the European market in December 2008.
Individual evidence
- ↑ a b entry on 5-azacytidine. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b Azacitidine data sheet from Sigma-Aldrich , accessed on March 9, 2011 ( PDF ).
- ↑ Entry on azacitidine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b c Vidaza specialist information ( Memento from September 21, 2011 in the Internet Archive ) (PDF; 244 kB).
- ↑ a b FDA press release on the Food and Drug Administration (FDA) website .
- ↑ Public Assessment Report (EPAR) of the European Medicines Agency (EMA) on: Vidaza .
Web links
- Entry for Azacitidine in the Human Metabolome Database (HMDB) , accessed November 7, 2013.
- Orphan drug list from the Association of Research-Based Pharmaceutical Manufacturers