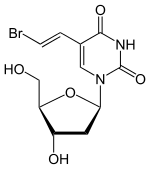
Brivudine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Brivudine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 11 H 13 BrN 2 O 5 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class |
Nucleoside analogs |
|||||||||||||||
| Mechanism of action |
Inhibition of viral DNA polymerase |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 333.135 g mol −1 | |||||||||||||||
| Melting point |
164-166 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Brivudine is a drug that can be used as an antiviral agent against herpes simplex type 1 and herpes zoster . It belongs to the group of nucleoside analogs. Compared to other nucleoside analogues ( acyclovir , valaciclovir , famciclovir ), it is characterized by a significantly (200- to 1000-fold) higher antiviral potency and a long half-life and intracellular residence time. The most common side effect is nausea, but overall side effects are rare.
history
Brivudine was produced in England and what was then the GDR in the 1970s . But it has only been used more widely since the indication was changed in 2001. Until 2000 it was approved for the treatment of herpes simplex infections. It has been used to treat herpes zoster since 2001 .
pharmacology
Mechanism of action
Brivudine is a thymidine - antimetabolite , it is effective against DNA viruses .
Brivudine is activated to triphosphate in several phosphorylation steps. Activation only takes place in the virus-infected cell because the process is catalyzed by a viral thymidine kinase . The triphosphates represent the actual active form. They lead to the inhibition of the viral DNA polymerase , to the incorporation of modified nucleobases into the DNA and consequently to the chain termination during the DNA elongation.
Brivudine triphosphate has a very long intracellular residence time of 10 hours, so that it also has enough time to develop its effect in the virus-infected cells. Since brivudine only inhibits virus replication, but does not damage the virus itself, it can only suppress the course of the infection. However, it cannot cause pathogen eradication and cannot prevent the recurrences typical of herpes viruses .
Spectrum of activity
Brivudine has high activity against herpes viruses. It is effective against herpes simplex type 1 and against varicella zoster viruses. In contrast, it is hardly effective against herpes simplex type 2 (e.g. genital infections). Brivudine is also effective against the Epstein-Barr virus in vitro . A case study on the treatment of EBV encephalitis also confirmed its effectiveness in vivo.
effectiveness
Brivudine is much more effective against herpes viruses than other nucleoside analogues (e.g. acyclovir). The time from the beginning of the treatment until the last appearance of new vesicles is used as the criterion. With acyclovir this time span is 18 hours, with brivudine 13 to 14 hours.
In addition, postherpetic neuralgia (= neuralgia following a herpes zoster disease) occurs significantly less frequently (approx. 25%) after treatment with brivudine than after treatment with acyclovir or famciclovir.
The significantly better effectiveness of brivudine compared to acyclovir is controversial. The arznei-telegram refers to possible conflicts of interest of the authors of the brivudine-favoring AWMF guideline and doubts the clinical relevance of the relevant studies.
Indications and dosage
Brivudine is indicated for herpes zoster and herpes simplex type 1. It is the drug of choice for this indication, especially in patients over 50 years of age.
It is used orally. The standard dose for adults is 125 mg per day for 7 days. The tablet should be taken at about the same time each day. No dose adjustment is necessary in patients with renal or hepatic insufficiency.
It is important for the success of the treatment that the therapy takes place within 72 hours of the onset of the skin symptoms (= phase of virus replication). It is advisable to start treatment later, as long as there are still fresh vesicles, if there are signs of visceral spread or in the case of florid zoster ophthalmicus and zoster oticus .
Side effects
Brivudine is usually well tolerated. The most common side effects affect the gastrointestinal tract: 2% of patients experience nausea. Diarrhea can also occur.
Rare side effects are:
- Nervous system disorders: headache, dizziness, etc.,
- Tiredness or trouble sleeping
- Increase in serum creatinine and urea (evidence of impaired kidney function),
- Hypersensitivity reactions of the skin,
- Changes in the blood count (reversible).
Interactions and restrictions on use
- Brivudine must not be administered at the same time or in the near future with the cytostatic 5-fluorouracil or other 5-fluoropyrimidines (e.g. tegafur ). At least 4 weeks must elapse between the administration of these drugs, since the metabolite of brivudine, bromovinyluracil , irreversibly inhibits the enzyme dihydropyrimidine dehydrogenase , which is responsible for the breakdown of pyrimidines . This leads to an accumulation and increased toxicity of 5-fluorouracil, combined with the risk of serious side effects: significant gastrointestinal disturbances, neutropenia and bone marrow depression . With the simultaneous administration of 5-fluorouracil and sorivudine , which like brivudine is broken down to the metabolite bromovinyluracil, this interaction has even led to deaths. This potentially fatal interaction with antineoplastic (5-fluorouracil, capecitabine , floxuridine, tegafur) or antifungal 5-fluoropyrimidines ( 5-fluorocytosine ) is in principle also important for brivudine.
- Another Rote-Hand-Brief was published dated May 12, 2020 with the following content: "Deaths may occur as a result of drug interaction between brivudine and fluoropyrimidines (e.g. fluorouracil (5-FU), capecitabine, tegafur, flucytosine). After completing brivudine treatment, there must be at least a four-week waiting period before treatment with a fluoropyrimidine can be started. In many cases, deaths occurred if this four-week waiting period was not adhered to (e.g. brivudine was used between two 5 -FU cycles taken). "
- Displacement of brivudine from plasma protein binding by other drugs that are also strongly protein bound.
Contraindications
- pregnancy and breast feeding period
- simultaneous administration of 5-fluorouracil (see above under interactions and restrictions of use)
- Immunosuppression
Cross resistance
There is cross-resistance to acyclovir in herpes simplex and varicella zoster viruses.
Pharmacokinetics
- Oral bioavailability: 30% (due to the pronounced first-pass effect )
- Absorption: 85%
- Terminal half-life: 16 hours
- Elimination: mainly renal, 20% via the faeces
Brivudine is almost completely absorbed enterally after oral administration. The maximum plasma level is reached after just 1 hour. Meals delay absorption, but do not reduce the absorption rate itself. During first-pass metabolism, about 65% of the inactive metabolite bromovinyluracil (BVU) is formed. This ineffective substance is produced by the enzyme pyrimidine phosphorylase, which splits off the sugar component. Brivudine is more than 95% bound to plasma proteins and the volume of distribution is 75 liters. If the kidney function is impaired, the half-life is significantly longer.
See also
literature
- Wehling, Martin - Clinical Pharmacology
- Karow, Thomas - Pharmacology and Toxicology
- Kojda, Georg - Pharmacology / Toxicology
- Austria Codex 2005/2006 Specialist information on Mevir ®
- Doctors newspaper, January 16, 2004
- Chemotherapy Journal. Issue 3/2003 HTML
- Österreichische Apothekerzeitung: Herpes zoster treated innovatively: Mevir ( Memento from March 26, 2009 in the Internet Archive ), issue 21/2003
Trade names
- Brivex (Switzerland)
- Mevir (Austria)
- Zostex (Germany, Austria)
Individual evidence
- ^ Entry on brivudine. In: Römpp Online . Georg Thieme Verlag, accessed on June 27, 2019.
- ↑ Data sheet (E) -5- (2-bromovinyl) -2′-deoxyuridine from Sigma-Aldrich , accessed on December 17, 2012 ( PDF ).
- ^ Epstein-Barr virus encephalitis after kidney transplantation and successful treatment with brivudine , accessed July 11, 2017.
- ↑ arznei-telegram (5/2007): Brivudine (Zostex) better than acyclovir (Zovirax aa)?
- ↑ Austria Codex specialist information 2005/2006 on Mevir®.
- ↑ ADR - Learning from mistakes - Potentially fatal interaction between brivudine (Zostex®) and 5-fluoropyrimidines (PDF; 36 kB), Deutsches Ärzteblatt, vol. 103, issue 27, July 7, 2006, accessed on the AkdÄ website.
- ↑ Rote-Hand-Brief from Berlin Chemie / Menarini dated August 27, 2012. (PDF) Retrieved September 4, 2012 .
- ↑ Yellow List Online: Rote-Hand-Brief on medicinal products containing brivudine | Yellow list. Retrieved May 12, 2020 .
All products are manufactured by Berlin Chemie AG . In Greece, Austria and Switzerland, they are sold by the parent company Menarini .

