Cesium iodide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
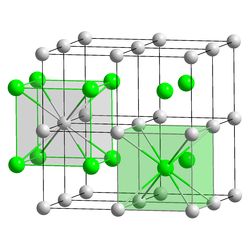
|
|||||||||||||||||||
| __ Cs + __ I - | |||||||||||||||||||
| Crystal system |
cubic |
||||||||||||||||||
| Space group |
Pm 3 m (No. 221) |
||||||||||||||||||
| Lattice parameters |
a = 4.5679 Å |
||||||||||||||||||
| Coordination numbers |
Cs [8], I [8] |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cesium iodide | ||||||||||||||||||
| other names |
Cesium iodide |
||||||||||||||||||
| Ratio formula | CsI | ||||||||||||||||||
| Brief description |
colorless and odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 259.83 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
4.51 g cm −3 |
||||||||||||||||||
| Melting point |
626 ° C |
||||||||||||||||||
| boiling point |
1280 ° C |
||||||||||||||||||
| solubility |
good in water (440 g l −1 at 20 ° C) |
||||||||||||||||||
| Refractive index |
1.7873 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−347 kJ mol −1 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Cesium iodide (also cesium iodide) is a white, crystalline salt with the empirical formula Cs I , which is used in IR , UV and X-ray spectroscopy . It is a salt of hydriodic acid . The material is very soft, difficult to polish and sensitive to humidity . The maximum application temperature is 200 ° C.
properties
Cesium iodide has a refractive index of 1.73916 and a reflection loss of 13.6% at a wavelength of 10.0 µm. It is translucent in the range 0.24–70 µm. The thermal conductivity is 1.13 W / (m · K) and the specific heat capacity is 201 J / (kg · K).
Cesium iodide is heat-stable up to approx. 600 ° C; thermal decomposition begins at 670–800 ° C.
The standard enthalpy of formation of cesium iodide is Δ H f 0 = −347 kJ / mol.
application
Cesium iodide can be used as a transparent scintillation material in scintillation counters. The high-energy radiation creates a so-called electromagnetic shower in the crystal , the photons of which are then converted into a measurable electrical signal with a photomultiplier . It can be used undoped or doped with thallium (Tl) or sodium (Na) as a scintillation material.
As an undoped material, it is also used as a beam splitter in IR spectroscopy .
See also
Individual evidence
- ↑ a b c d data sheet cesium iodide from AlfaAesar, accessed on February 16, 2010 ( PDF )(JavaScript required) .
- ↑ Entry on cesium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ^ CRC: Handbook of Chemistry and Physics . 55th edition. CRC-Press, 1974, ISBN 0-87819-454-1 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Inorganic Crystals, pp. 10-246.
- ↑ a b Entry on cesium iodide in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1170.
- ^ IA Kulikov, ML Malyshev: Radiation-chemical decomposition of CsI . In: Atomic Energy 1983 , 55 (5), pp. 316-318. doi : 10.1007 / BF01123994
- ↑ E. Riedel: Inorganic Chemistry . 8th edition, de Gruyter, Berlin 2011, ISBN 9783110225679 . P. 624.
Web links
- Korth Kristalle GmbH: Cesium iodide


