Fibroblast growth factor
The fibroblast growth factors (abbreviation FGF , from English Fibroblast Growth Factor ) are a group of growth factors that is referred to as the FGF family. A total of 23 members of the FGF group are known to date: FGF-1 to FGF-23.
FGFs are single-chain polypeptides with a mass mostly between 16 and 22 kDa . They belong to the signal proteins that are important and potent regulators of cell growth and differentiation of cells . They play a key role in embryonic development . Correspondingly, disorders of the FGF functions lead to severe developmental disorders in the embryonic period. In the adult organism, FGFs control tissue repair processes and are actively involved in the processes of wound healing and the formation of new vessels ( angiogenesis ), as well as in the regeneration of nerves and cartilage tissue. FGFs have been detected in almost all tissues in the organism.
FGFs control and change (usually stimulate) the proliferation (reproduction), migration (migration) and differentiation of cells , in particular of endothelial cells , but also of muscle cells (especially smooth muscle cells ) and fibroblasts . The complex process of angiogenesis is essentially controlled and influenced by growth factors of the FGF family. FGF-1 (acidic-FGF) and FGF-2 (basic-FGF) are prototypes of the FGF family.
Mechanism of action
FGF molecules bind to their specific receptors (FGFR = FGF receptor) on the cell surface. FGFRs are receptor tyrosine kinases which - after binding the ligand FGF - are activated by auto- phosphorylation and initiate a signal cascade with subsequent gene activation intracellularly . FGFRs are composed of an extracellular region, the three immunoglobulin-like ( IG-like ) protein domains have (D1-D3), a singular transmembrane helix , and an intracellular domain with protein tyrosine - kinase activity. There are four FGFRs: FGFR1, FGFR2, FGFR3, FGFR4. By alternative mRNA - splicing the receptors FGFR1-3 additional forms of FGFRs result (overall there are seven known FGFRs), which are denoted by "b" and "c". FGF-1 is the only ligand that binds to all seven receptors on the cell surface. The actual signal complex on the cell membrane that arises after binding of FGF and FGFR is referred to as the ternary complex , which consists of two identical FGF ligands, two identical FGF receptor units and either one or two heparan sulfate chains.
A special property of the mechanism of action of the FGFs is that it is significantly enhanced by the particularly high affinity of the FGFs for proteoglycans , heparan sulfates and heparin (glycosaminoglycan). For this reason, the growth factors of the FGF family were previously also referred to as heparin-binding growth factors (HBGFs).
Physiologically, FGFs are actively secreted in any form of tissue damage, but especially in hypoxia and ischemia ( up-regulation ).
FGF family
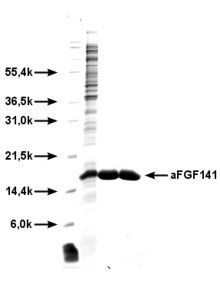
FGF-1 (a-FGF) is the most active growth factor in the FGF family. It consists of 141 amino acids . The gene coding for FGF-1 is located on chromosome 5 . Due to its extensive binding capacity with all FGF receptors, the biological, mitogenic cell effects are particularly pronounced and characterized by the initiation of cell proliferation , migration and differentiation . FGF-1 has a particular effect on endothelial cells , but also on many other cell types. Due to the particularly pronounced angiogenic activity of FGF-1, FGF-1 has recently been investigated more intensively in clinical research and used in various clinical studies in human medicine . The plasma half-life of FGF-1 after intramyocardial injection is between 0.4 and 4.6 hours.
FGF-2 (b-FGF) has a similar molar mass as FGF-1; the structure is more than 50% identical to that of FGF-1. The gene coding for FGF-2 is located on chromosome 4 . The effects of FGF-2 are similar to those of FGF-1, but not quite as intense. It is also formed by adipocytes , among other things , and influences bone metabolism.
FGF-3 consists of 240 amino acids, its structure is approx. 40% homologous with FGF-1; the coding gene is located on chromosome 11 . The physiological effects of FGF-3 are still poorly understood, but FGF-3 may be particularly important during the embryonic period.
FGF-4 (formerly K-FGF or hst1) consists of 206 amino acids, is 40% homologous with the structure of FGF-1-3, and the coding gene is on chromosome 11. FGF-4 is often found in tumors , especially in stomach tumors. FGF-4 occurs only in low concentrations in healthy adult tissues.
FGF-5 consists of 251 amino acids, the coding gene is located on chromosome 4 . FGF-5 apparently plays an important (including angiogenic) role during embryonic development, but FGF-5 is only found in very low concentrations in adult tissues.
FGF-6 (formerly hst2) is 70% homologous with FGF-4. The coding gene is located on chromosome 12 . Little is known about its effects; FGF-6 may play a role in wound healing .
FGF-7 was first named Keratinocyte Growth Factor (KGF); it has a special proliferative effect on epithelial cells . The coding gene is on chromosome 15 .
FGF-8 (gene localization on chromosome 10 ) may play a key role in the formation of the extremities during the embryonic period.
FGF-9 , initially referred to as glioma-derived growth factor (GDGF), particularly stimulates the proliferation and activation of glial cells in the brain .
FGF-10 through FGF-22 : Although the structures and amino acid sequences of these growth factors are described, little is known about the detailed functions of these proteins . FGF-18 stimulates the formation of cartilage in model organisms upon intra-articular injection . A recombinantly produced human FGF-18 is in clinical testing .
FGF-23 is secreted by osteocytes and is an important regulator of the phosphate and vitamin D balance. FGF-23 stimulates the excretion of phosphate by the kidneys . The task of FGF-23 is to keep the phosphate level in the blood constant despite the different phosphate intake with food. Increased blood levels of FGF-23 lead to a decrease in the phosphate level in the blood ( hypophosphataemia ), reduced production of 1,25 (OH) 2 -vitamin D and rickets or bone softening (osteomalacia) . Decreased blood levels of FGF-23 lead to increased phosphate levels in the blood ( hyperphosphataemia ), increased production of 1,25 (OH) 2 -vitamin D, soft tissue calcifications , excessive bone formation ( hyperostosis ) and reduced life expectancy. In kidney patients who have to start dialysis treatment , increased FGF-23 levels are associated with increased mortality .
history
The first FGFs were discovered and their chemical structures described in the 1970s. At first it was assumed that they only act on fibroblasts (hence the name). However, it was later found that FGFs have much more general functions - especially proliferation and differentiation - and can act on almost all cells. Today even FGFs are known that have no effect on fibroblasts, e.g. B. FGF-7 and FGF-9. FGF-1 and FGF-2 were first obtained from the brain of cattle and isolated; later the structures of the human growth factors FGF-1 and FGF-2 were also described.
The strengthening effect of heparin and heparan sulfates on the function of the FGFs was recognized at an early stage . To date (2007) 23 different sub-types of the FGF family have been described.
Functions and Medical Significance
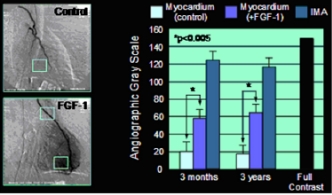
The different FGF types have intense mitogenic activities and are of great importance for organ differentiation and development in the embryonic period. They regulate cell proliferation, migration and differentiation . Regular cell and tissue differentiation without FGFs is not possible. In adult tissues and organs, FGFs - especially FGF-1 - have an extremely intense activity with regard to the induction of angiogenesis. This property of FGFs has recently aroused the interest of medical research, since angiogenesis can be used as a therapeutic principle in those disease states and disorders in which there is a disorder of the arterial blood flow ( atherosclerosis ), e.g. B. Coronary artery disease (CHD) and peripheral arterial occlusive disease (PAOD). Hypoxia and ischemia trigger the secretion of FGF-1 and FGF-2, so that the FGF receptors in the tissue are up-regulated . The induction of angiogenesis caused by the binding of FGF and FGFR can be understood in terms of a repair process that improves blood flow. Clinical studies with patients suffering from severe coronary artery disease have shown FGF-1-induced new vessels in the human heart muscle, as well as a local increase in blood flow with a reduction in angina pectoris symptoms. Even with disorders of wound healing , e.g. B. in diabetic ulcers, FGFs, especially FGF-1, promote wound healing. Animal experiments have even shown that FGF-1 has a significantly reducing effect on the extent of a stroke .
Example of FGF-1 induced angiogenesis in human heart muscle. Left, angiographic representation of the newly formed vascular network in the area of the anterior wall of the left ventricle. Right, gray value analysis to quantify the angiogenic effect.
Stress SPECT analysis of the human heart muscle after intramyocardial FGF-1 application. Left, before FGF-1 treatment. Right, three months after treatment.
Due to the broad mitogenic activity of FGFs, they are also being investigated in current clinical research with regard to their positive effect on osteoporosis (activation of osteoblasts by FGF-1) and with regard to their repair potential in cartilage damage ( osteoarthritis ). Also, FGF-1 and (lower) and FGF-2 may be a stimulation of the so-called heart - progenitor cells ( cardiac progenitor cells ) causing these local, existing in the myocardium, the heart muscle progenitor cells into adult cardiomyocytes in terms of maturation. Finally, another clinical potential of FGF-1 lies in its ability to regenerate nerve cells .
Since FGFs are also found in high concentrations in many tumors and are involved in tumor angiogenesis there, research on anti-angiogenic tumor therapy is also aimed at inhibiting the angiogenic activity of FGFs.
Individual evidence
- ↑ a b G. M. Rubanyi (editor): Angiogenesis in health and disease. M. Dekker, Inc., New York - Basel, 2000
- ↑ PL Lee et al .: Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. In: Science 245, 1989, pp. 57-60. PMID 2544996
- ^ DM Ornitz et al: Receptor Specificity of the Fibroblast Growth Factor Family. In: J Biol Chem 271, 1996, pp. 15292-15297. PMID 8663044
- ↑ DM Ornitz and N. Itoh: Fibroblast growth factors. In: Genome Biol 2, 2001, pp. 1-12. PMID 11276432 , PMC 138918 (free full text)
- ↑ Blaber, M., DiSalvo, J. Thomas, KA: X-ray crystal structure of human acidic fibroblast growth factor. In: Biochemistry 35, 1996, pp. 2086-2094. PMID 8652550
- ↑ a b T. J. Stegmann: A human growth factor in the induction of neoangiogenesis. In: Exp Opin Invest Drugs 7, 1998, pp. 2011-2015. PMID 15991943
- ↑ Kühn MC, Willenberg HS, Schott M, Papewalis C, Stumpf U, Flohé S, Scherbaum WA, Schinner S: Adipocyte-secreted factors increase osteoblast proliferation and the OPG / RANKL ratio to influence osteoclast formation . In: Mol Cell Endocrinol . 349, No. 2, 2012, pp. 180-188. doi : 10.1016 / j.mce.2011.10.018 . PMID 22040599 .
- ↑ Shiguang Liu and L. Darryl Quarles: How Fibroblast Growth Factor 23 Works . In: J Am Soc Nephrol . No. 18 , 2007, p. 1637-1647 ( Article ).
- ↑ OM Gutierrez et al .: Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis . In: N Engl J Med . No. 359 , 2008, p. 584-592 ( abstract ).
- ↑ D. Gospodarowicz et al: Purification of a growth factor for ovarian cells from bovine pituitary glands. In: PNAS 71, 1974, pp. 2295-2299. PMID 4526208 . PMC 388439 (free full text)
- ↑ D. Gospodarowicz: Localization of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. In: Nature 249, 1974, pp. 123-127. PMID 4364816
- ↑ D. Gospodarowicz: Purification of a fibroblast growth factor from bovine pituitary. In: J Biol Chem 250, 1975, pp. 2515-2520. PMID 1168187
- ↑ G. Gimenez-Gallego et al: The complete amino acid sequence of human brain-derived acidic fibroblast growth factor. In: Biochem Biophys Res Commun 138, 1986, pp. 611-617. PMID 3527167
- ↑ G. Gimenez-Gallego et al .: Human brain-derived acidic and basic fibroblast growth factors: amino terminal sequences and specific mitogenic activities. In: Biochem Biophys Res Commun 135, 1986, pp. 541-548. PMID 3964259
- ↑ SC Thornton et al.: Human endothelial cells: use of heparin in cloning and long-term serial cultivation. In: Science 222, 1983, pp. 623-625. PMID 6635659
- ^ J. Folkman: Angiogenic therapy of the heart. In: Circulation 97, 1998, pp. 628-629. PMID 9495294
- ↑ M. Simons et al .: Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. In: Circulation 102, 2000, pp. E73-E86. PMID 10982554
- ↑ R. Khurana and M. Simons: Insights from angiogenesis trials using fibroblast growth factor for advanced arteriosclerotic disease. In: Trends Cardiovasc Med 13, 2003, pp 116-122. PMID 12691676
- ^ TJ Stegmann, and T. Hoppert: Combined local angiogenesis and surgical revascularization for coronary heart disease. In: Current Intervent Cardiol Reports 1, 1999, pp. 172-178. PMID 11096622
- ↑ TJ Stegmann et al.: Therapeutic angiogenesis: intramyocardial growth factor delivery of FGF-1 as sole therapy in patients with chronic coronary artery disease. (PDF; 449 kB) In: CVR 1, 2000, pp. 259-267.
- ↑ LE Wagoner et al: Angiogenesis Protein Therapy With Human Fibroblast Growth Factor (FGF-1): Results Of A Phase I Open Label, Dose Escalation Study In Subjects With CAD Not Eligible For PCI Or CABG. In: Circulation 116, 2007, p. 443.
- ↑ JMA Laird et al: Acidic Fibroblast growth factor stimulates motor and sensory axon regeneration after sciatic nerve crush in the rat. In: Neuroscience 65, 1995, pp. 209-216. PMID 7538644
- ^ J. Folkman: Fighting cancer by attacking its blood supply. In: Sci Am 275, 1996, pp. 150-154. PMID 8701285