2-methyl-1-propanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-methyl-1-propanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 10 O | |||||||||||||||
| Brief description |
colorless liquid with a fusel oil odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 74.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.80 g cm −3 |
|||||||||||||||
| Melting point |
−108 ° C |
|||||||||||||||
| boiling point |
108 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.3955 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Methyl-1-propanol (also i- butanol or isobutanol) belongs to the group of alkanols , which in turn belong to the alcohols . Isobutanol comes e.g. B. in fusel oil .
Extraction and presentation
Isobutanol and 1-butanol are obtained chemically by hydroformylation or hydrocarbonylation of propene . In contrast to the production of 1-butanol, you continue to work with the other reaction product and hydrogenate it:

- Propene reacts with carbon monoxide and hydrogen to form butanal and 2-methylpropanal .
Isobutanol can be produced biotechnologically with the help of microorganisms . Then it serves as a regenerative biofuel, similar to 1-butanol .
properties
Physical Properties
2-methyl-1-propanol is a colorless liquid that smells sweet. Like all butanols, 2-methyl-1-propanol is also flammable. Can be 2-methyl-1-propanol with all common organic solvents such as ether , ethylene glycol , alcohols , ketones and aldehydes mix as desired, in water , however, it is only sparingly soluble.
The compound forms azeotropically boiling mixtures with a number of other solvents . The azeotropic compositions and boiling points can be found in the following table. No azeotropes are formed with methanol , ethanol , n-propanol , 2-propanol , n-butanol , 2-butanol , ethanediol , chloroform , acetone , dibutyl ether , ethyl acetate , isopropyl acetate , n-butyl acetate , DMSO , acetonitrile and carbon disulfide .
Azeotropes with various solvents solvent water n-hexane n-heptane Cyclohexane Dioxane Content of 2-methyl-1-propanol in % 67 2 27 14th 4th boiling point in ° C 89 68 91 78 101
solvent benzene toluene Ethylbenzene Xylene Methyl isobutyl ketone Content of 2-methyl-1-propanol in % 8th 45 80 88 91 boiling point in ° C 79 101 107 107 108
Chemical properties
- Reactions
Possible reactions of 2-methyl-1-propanol are esterification to an ester , dehydrogenation to an aldehyde , oxidation to a carboxylic acid and the elimination of water to form an alkene .

- 2-Methyl-1-propanol reacts with acetic acid to form isobutyl acetate and water.

- Dehydration of 2-methyl-1-propanol to 2-methylpropanal .
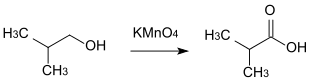
- 2-methyl-1-propanol is oxidized to 2-methylpropanoic acid.
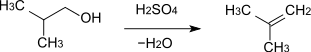
- 2-Methyl-1-propanol reacts to 2-methylpropene with elimination of water .
Safety-related parameters
2-methyl-1-propanol forms highly flammable vapor-air mixtures. The compound has a flash point of 27 ° C. The explosion range is between 1.7% by volume (52 g / m 3 ) as the lower explosion limit (LEL) and 11% by volume (340 g / m 3 ) as the upper explosion limit (UEL). Correlating the explosion limits with the vapor pressure function results in a lower explosion point of 26 ° C and an upper explosion point of 59 ° C. The maximum explosion pressure is 8.5 bar. The limit gap width was determined to be 0.94 mm (50 ° C). This results in an assignment to explosion group IIA. The ignition temperature is 430 ° C. The substance therefore falls into temperature class T2.
use
2-Methyl-1-propanol is used in derivatives as a solvent for the synthesis of plasticizers and esters , which are used as fragrances and aromas, but also as solvents, thinners and additives in nitrocellulose lacquers, synthetic resins, cleaning agents, printing inks and in fuel. It is mainly used in the paint industry and improves the properties of paints. It is also being used as a fuel in long-distance motorsport tests.
Individual evidence
- ↑ Entry on 2-METHYLPROPANOL in the CosIng database of the EU Commission, accessed on February 25, 2020.
- ↑ a b c d e f g h i j k l m n o p q r s t Entry on 2-methyl-1-propanol in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ a b c d Toxicological assessment of 2-methylpropanol-1 (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on May 1, 2018.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-370.
- ↑ Entry on 2-methylpropan-1-ol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 78-83-1 or 2-methyl-1-propanol ), accessed on November 2, 2015.
- ↑ Alexandra M. Goho: Bacteria produce butanol ; Technology Review ; January 30, 2008.
- ↑ a b I. M. Smallwood: Handbook of organic solvent properties. Arnold, London 1996, ISBN 0-340-64578-4 , pp. 12-13.
- ↑ a b c d e E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ a b D. Stoye: Solvents. In: Ullmann's Encyclopedia of Technical Chemistry . Wiley-VCH Verlag, Weinheim 2012, doi : 10.1002 / 14356007.a24_437 .



