Chalcosine
| Chalcosine | |
|---|---|
| Chalcosine specimen from the "Mammoth Mine", Mount Gordon , Mount Isa , Queensland, Australia (size: 3.5 × 3.3 × 2.1 cm) | |
| General and classification | |
| other names |
|
| chemical formula | Cu 2 S |
|
Mineral class (and possibly department) |
Sulphides, sulphosalts - metal: sulfur, selenium, tellurium> 1: 1 |
|
System no. to Strunz and to Dana |
2.BA.05a ( 8th edition : II / B.01) 04/02/07/01 |
| Crystallographic Data | |
| Crystal system | monoclinic |
| Crystal class ; symbol | monoclinic prismatic; 2 / m |
| Space group | P 2 1 / c (No. 14) |
| Lattice parameters |
a = 15.25 Å ; b = 11.88 Å; c = 13.49 Å β = 116.3 ° |
| Formula units | Z = 48 |
| Frequent crystal faces | {110}, {010}, {001}, {111}, {112}, {113}, {023} |
| Twinning | Crossing twins according to (112) and triplets according to (110) |
| Physical Properties | |
| Mohs hardness | 2.5 to 3 |
| Density (g / cm 3 ) | measured: 5.5 to 5.8; calculated: 5.80 |
| Cleavage | indistinct after {110} |
| Break ; Tenacity | shell-like |
| colour | lead gray, steel gray, matt black |
| Line color | dark gray |
| transparency | opaque |
| shine | Metallic luster |
Chalcosine , outdated also known as copper luster or copper glass , is a frequently occurring mineral from the mineral class of " sulfides and sulfosalts " with the composition Cu 2 S (also α-Cu 2 S) and is chemically a copper (I) sulfide .
Chalcosine crystallizes in the monoclinic crystal system , is opaque in every form and usually develops prismatic, tabular and, due to the formation of twins , pseudo-hexagonal crystals . It can also be found in the form of granular to massive aggregates . Fresh samples are initially lead-gray to steel-gray in color and metallic luster . However, over time they blacken and become dull.
Etymology and history
The mineral was named Chalkosin in 1832 by François Sulpice Beudant , who derived the name from the Greek word χαλκός chalkos for copper.
However, the mineral was already known in the 16th century under the mining name copper glass and later as copper luster .
classification
Already in the outdated 8th edition of the mineral classification according to Strunz , the chalcosin belonged to the mineral class of "sulfides and sulfosalts" and there to the department of "sulfides etc. with [the substance ratio] M: S> 1: 1", where it together with Djurleit , Berzelianite and Weissite the “Chalcosine-Berzelianite group” with the system no. II / A.01 .
In the Lapis mineral directory according to Stefan Weiß, which, out of consideration for private collectors and institutional collections, is still based on this old form of Karl Hugo Strunz's system , the mineral was given the system and mineral number. II / B.01-10 . In the “Lapis system” this corresponds to the section “Sulphides, selenides and tellurides with [the] ratio metal: S, Se, Te> 1: 1”, where chalcosine together with anilite , diginite , djurleit , geerite , roxbyite , spionkopite and Yarrowit forms the group of "copper sulfides" (as of 2018).
The 9th edition of Strunz's mineral systematics , which has been valid since 2001 and updated by the International Mineralogical Association (IMA) until 2009, also assigns chalcosine to the category of "Metal sulfides, M: S> 1: 1 (mainly 2: 1)" . This is, however, further subdivided according to the predominant metals in the compound, so that the mineral can be found according to its composition in the sub-section "with copper (Cu), silver (Ag), gold (Au)", where it is the only member unnamed group forms 2.BA.05a .
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns chalcosin to the class of "sulphides and sulphosalts" and there in the category of "sulphide minerals". Here he is together with Djurleit, Digenit, Roxbyit, Anilith, Geerit and Spionkopit in the " chalcosine group (formula: Cu 2-x S) " named after him with the system no. 04/02/07 within the subsection "Sulphides - including selenides and tellurides - with the composition A m B n X p , with (m + n): p = 2: 1".
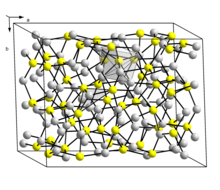
Crystal structure
Chalcosine, more precisely deep chalcosine (also deep copper luster ) crystallizes monoclinically in the space group P 2 1 / c (space group no. 14) with the lattice parameters a = 15.25 Å ; b = 11.88 Å; c = 13.49 Å and β = 116.3 ° and 48 formula units per unit cell .
At a temperature of over 103 ° C, deep chalcosine changes into the hexagonal modification and is accordingly referred to as high chalcosine or high copper luster . The space group of high chalcosine is P 6 3 / mmc (space group no. 194) and the lattice parameters are a = 3.95 Å and c = 6.75 Å with 2 formula units per unit cell .
Education and Locations

As a frequent mineral formation, chalcosine can be found in many localities, which are mainly divided into two types of formation:
In transition - and displacement - deposits , more rarely, in pegmatitic - pneumatolytic deposits that (of rising ascendant ) hydrothermal solution be penetrated, is chalcocite is mostly in paragenesis with bornite , Enargite , various Fahl ore , pyrite and other sulfides.
Well-known deposits of this type include Butte (Montana) in the USA, Schesqasghan (formerly Džezkazgan ) in Kazakhstan, Tsumeb in Namibia, Musina (formerly Messina ) in South Africa and the "Turjinskii Mine" (Turginsk Mine) on the Turja River in the Northern Urals in Russia.
In descending (descending) sedimentation and cementation zones, chalcosine often accumulates through the precipitation of solutions containing copper sulphate, whereby other sulphides are displaced. Many of these ore-rich cementation zones and vein deposits have now been mined, especially in Europe and the USA, and the mining of rather poor porphyry copper ore deposits is often only economically feasible with secondary enrichment. Well-known deposits here include the Bingham Canyon Mine (Utah) and Bisbee (Arizona) in the USA and Cerro de Pasco in Peru. Of great importance is the Kupferschiefer at are Mansfeld - Sangerhausen in Germany, the copper marl around Legnica in Poland, Qonyrat ( Kounrad ) in Kazakhstan, Olmaliq ( Almalyk ) in Uzbekistan and the Central Asian Altai Mountains .
In the oxidation zone , however, chalcosine is not stable and is replaced either by solid copper , by the sulfide Covellin , by the oxide cuprite or the carbonates azurite and malachite .
As a frequent mineral formation, chalcosine can be found in many places, whereby so far (as of 2012) around 4500 locations are known, including on the Antarctic Peninsula , in rock samples from the Mid-Atlantic Ridge and East Pacific Ridge .
The "M'Sesa Mine" near Kambove ( Katanga Province ) in the Democratic Republic of the Congo, where crystals up to 25 cm in size have been found, are also known for the extraordinary finds of chalcosin . Well-formed crystals several centimeters in diameter also occurred at Redruth and St Just (England) in the United Kingdom, Bristol (Connecticut) and in the "Flambeau Mine" near Ladysmith (Wisconsin) in the USA.
use
Chalcosine was an important raw material for the extraction of copper until the most productive mining areas in England and the USA were exhausted . Today, besides other copper sulfides such as bornite and covelline , chalcosine still plays an important role as an ore mineral in stratified deposits such as B. Lubin in Lower Silesia, Poland.
See also
literature
- Martin Okrusch, Siegfried Matthes: Mineralogy. An introduction to special mineralogy, petrology and geology . 7th, completely revised and updated edition. Springer, Berlin [a. a.] 2005, ISBN 3-540-23812-3 , pp. 32 .
- Helmut Schrätze , Karl-Ludwig Weiner : Mineralogy. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 118-125 .
Web links
- Chalcosine. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on May 4, 2020 .
- Chalcocite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed on May 4, 2020 .
- American-Mineralogist-Crystal-Structure-Database - Chalcocite. In: rruff.geo.arizona.edu. Retrieved May 4, 2020 .
Individual evidence
- ↑ Malcolm Back, William D. Birch, Michel Blondieau and others: The New IMA List of Minerals - A Work in Progress - Updated: March 2020. (PDF; 2.44 MB) In: cnmnc.main.jp. IMA / CNMNC, Marco Pasero, March 2020, accessed May 1, 2020 .
- ^ David Barthelmy: Chalcocite Mineral Data. In: webmineral.com. Retrieved May 4, 2020 .
- ↑ a b c d e Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 62 .
- ^ A b Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 119 .
- ↑ a b Chalcocite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 (English, handbookofmineralogy.org [PDF; 66 kB ; accessed on May 4, 2020]).
- ^ FS Beudant: Chalkosine, cuivre sulfuré . In: Traité Élémentaire de Minéralogie . tape 2 , 1832, p. 408–410 ( rruff.info [PDF; 119 kB ; accessed on May 4, 2020]).
- ↑ Hans Lüschen: The names of the stones. The mineral kingdom in the mirror of language . 2nd Edition. Ott Verlag, Thun 1979, ISBN 3-7225-6265-1 , p. 260 (as copper luster).
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF; 1.82 MB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed May 4, 2020 .
- ↑ a b Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 297-298 .
- ^ A b Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 122 .
- ↑ Chalcocite. In: mindat.org. Hudson Institute of Mineralogy, accessed May 4, 2020 .
- ↑ Find location list for chalcosine in the Mineralienatlas and Mindat , accessed on May 4, 2020.
- ↑ Petr Korbel, Milan Novák: Mineral Encyclopedia (= Dörfler Natur ). Edition Dörfler im Nebel-Verlag, Eggolsheim 2002, ISBN 978-3-89555-076-8 , p. 20 .