Optical activity

The grains contrast lightly against the background, since strength is optically active, i.e. rotates the direction of polarization. The grains show dark crosses parallel to the directions of the filters. This is because the starch in these grains forms radially oriented, uniaxially birefringent crystals.
The optical activity is a property of some transparent materials to rotate the polarization direction of the light . When linearly polarized light passes through an optically active medium , the plane of polarization of the light is rotated a little on each molecule . In the case of chiral molecules, this effect that occurs on every single molecule does not average out to zero with statistical certainty, so that the single rotations can accumulate. After the light has passed through the entire body of the substance, there is a large measurable net amount of rotation.
A distinction is made between clockwise (polarization plane clockwise on the observer side) and counterclockwise substances. A third category consists of racemates in which the same concentrations of the two dextrorotatory and levorotatory substances ( enantiomers ) are always present and which are therefore optically inactive .
Chemical-physical causes
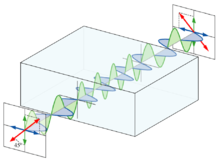
Polarized light
Light is an electromagnetic wave . In such an electric field oscillates - i. H. the field vector , which describes the field strength and direction - perpendicular to the wave vector (direction of propagation) of the wave. Due to the oscillation of the vector and the direction of propagation, a very specific level in space is characterized. If you look towards the beam, you can only see the "edge" of this plane, which is inclined at a certain angle.
Normal light contains rays that oscillate in any direction, whereas linearly polarized light oscillates only in one plane. In optically active substances the inclination of this plane is changed. This means that normal light is also rotated by optically active substances, but it is not noticeable because all directions are represented before and after. In contrast, with polarized light, the change in angle can be measured directly.
Rotation of the polarization direction
In order to understand the phenomenon of optical activity, one must first understand why most substances are not optically active. Because every molecule of every compound contains centers of charge and thus an electric field that interacts with the wave and can easily rotate the plane of vibration . The degree of this rotation depends crucially on the spatial orientation of the molecule to the shaft. The exact mirror image of a molecule (the enantiomer ) exactly reverses a rotation that has taken place.
In a solution the molecules are statistically distributed in every possible position due to the thermal movement . So you can say that a beam rotated by a molecule will hit a molecule that is rotated to exactly match the mirror image of the first and will reverse the rotation. In general, substances are therefore not optically active. If enantiomers are present in the same amount (1: 1 mixture) and the rotation of the polarized light is canceled, it is called a racemate .
The reason for the optical activity of chiral substances lies precisely in the mirror image idea : by definition, they can not be brought into congruence with their mirror image by rotation, i.e. This means that it is converted into its mirror image by turning, which means that no mirror images are present in the pure enantiomer and the rotation cannot therefore be exactly reversed. This actually results in a macroscopic rotation of the polarization.
Rotations are conventionally indicated with a positive sign (+) and referred to as right if they occur clockwise when looking against the direction of propagation. The fabrics are called clockwise or counterclockwise. There is no permanent, i. H. for all substances applicable correlation between the ( R ) - or ( S ) - configuration (or the D - or L configuration) of enantiomers and the direction of rotation of the linearly-polarized light.
If two oppositely polarized waves of the same frequency are superimposed , a linearly polarized wave is created. The opposite occurs in optical activity: a linearly polarized wave is split into two circularly polarized waves, one that rotates to the left and one that rotates right. Optically active substances now have the property that one of these two waves has a higher speed of propagation . At the end of the crystal , one wave has not rotated as far as the other, the superposition of both waves again results in a linearly polarized wave (the frequency is not changed in the medium), whose electric field vector is rotated by an angle alpha.
Specific angle of rotation
The macroscopic angle of rotation that is found when linearly polarized light passes through an optically active substance depends initially on the substance itself; H. different molecules affect light differently. In addition, the angle of rotation is influenced by the following factors:
- of the wavelength of light, even Optical rotatory dispersion (ORD) called
- on the temperature of the sample, which determines the thermal movement of the individual molecule
- on the number of molecules that are passed by the light, i.e. on the concentration of a sample and the length that the light travels through the sample
- from the solvent , if it is a solution.
If the wavelength λ of the light and the temperature T are given, the specific angle of rotation α of a substance (for this wavelength and this temperature) can be determined:
With
- the measured (unspecific) angle of rotation
- the mass concentration of the solution
- the penetrated thickness .
In tables, the angle is usually given for yellow sodium light ( λ = 589 nm or “D” for the sodium D line ) and a temperature of 20 ° C (or 25 ° C) with the symbol (or ).
Literature values refer to the otherwise rather unusual units (corresponds to 1 g of substance per 100 cm³ of solution) and d = 1 dm.
To
with the circular wave number , the angle of rotation depends reciprocally on the square of the wavelength, provided that a wavelength range is considered in which the optically active substance does not absorb light . In contrast, the Cotton effect dominates in the area of absorption maxima .
Other causes
Most crystals also rotate the light, u. a. Quartz , calcite , cinnabar and sodium chlorate . With them, the asymmetry lies in the crystal structure .
In the presence of a static magnetic field , all molecules are optically active. This Faraday effect was one of the first discoveries that showed a connection between light and electromagnetism .
Rotation angle
The angle of rotation by which the plane of polarization of linearly polarized light of the wavelength was rotated after passing through the distance is:
where the refractive index is for left-handed and that for right-handed light.
Practical meaning
The optical activity can be measured using a polarimeter . The above formula can be used to calculate the concentration of a solution from the measured angle of rotation, which is particularly useful in sugar processing ( saccharimetry ). Some historical names can be traced back to this practical application:
- Dextrose ( Latin dexter , right) 'is a historical name for glucose , which is clockwise.
- Laevulose (Latin laevus 'links') is a historical name for fructose , which is left-rotating.
- Invert sugar is a mixture of glucose and fructose that is created when sucrose is broken down. The direction of rotation is inverted from right to left , the new angle of rotation is the sum of the angles of rotation of the glucose and fructose present in the same concentration.
Natural products often have a large number of chiral centers with a unique configuration and are therefore in the form of enantiomers. These rotate the plane of polarized light by the same amount in opposite directions (different signs ) and usually have different physiological effects on living organisms . Therefore, measuring the optical purity with a polarimeter is an important quality criterion for chiral drugs .
literature
- Eugene Hecht: Optik , Oldenbourg, 4th edition 2005, ISBN 3-486-27359-0 , p. 581 ff.
See also
- electro-optical Kerr effect (electrically induced polarization rotation in the medium)
- Magneto-optical Kerr effect (polarization rotation of light in reflection)
- LCD
- Resolution






![{\ displaystyle \ left [\ alpha \ right] _ {\ lambda} ^ {T} = {\ frac {\ alpha} {\ beta \ cdot d}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7506ec4f6e552850a822872c6eccda7368e95738)


![{\ displaystyle \ left [\ alpha \ right] _ {D} ^ {20}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/63e05cbfb37b9d156add3fb13f33c090db54f53a)
![{\ displaystyle \ left [\ alpha \ right] _ {D} ^ {25}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0d6f2cd86b58671dc333498bf84103c824d9afc4)

![{\ displaystyle [\ alpha] = {\ frac {k} {\ lambda ^ {2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/97a71f49534e08edd1ec35e01e3a73d8782b32af)




