Polyferrocenes
Polyferrocenes are a class of ferrocene- containing polymers that can be divided into several subclasses. As a component of macromolecular chemistry, ferrocene offers many advantages over pure hydrocarbons . It is temperature-stable, resistant to acids and alkalis, is stable in air and can be easily produced from inexpensive raw materials. The wide range of possible variations of the substitution on the ferrocene base makes it possible to produce polymers with interesting electronic and photonic properties. Many polyferrocenes are relatively easily accessible. Poly (1,1'-ferrocene-silane) s, for example, can be produced by ring-opening polymerization and have a large number of interesting material characteristics, such as a high refractive index or semiconductor properties .
Nomenclature and classification
Polyferrocenes can be divided into three subclasses. The compounds of the first subclass consist of 1,1'-ferrocene fragments which are connected via different spacers . These include poly (1,1'-ferrocene-alkylene) s, consisting of ferrocene and Alkyeinheiten are constructed and poly (1,1'-ferrocene-arylene) s, in which 1,1'-fragments by spacer such as aryl moieties are connected . There are also polyferrocenes in which the cyclopentadienlyl units are linked via phosphane , silane or sulfur spacers . Depending on the heteroatom, these are called poly (1,1'-ferrocene-phosphane) e, poly (1,1'-ferrocene-silane) e or poly (1,1'-ferrocene-sulfide) e. There are also polyferrocenes with bridges that are formed by other main or subgroup elements such as gallium , selenium or tin .
If the cyclopentadienyl ligands of the ferrocene units are directly linked to one another, they form a further subclass; the corresponding polymetallocenes are called poly (1,1'-ferrocene) s.
In molecules of the third subclass, the cyclopentadienyl radical is substituted with polymerizable side chains such as 1,3-butadiene . These side chains can be polymerized radically or anionically or copolymerized with other monomers . In these cases, polymers such as poly (1-ferrocene-1,3-butadiene) arise, in which the ferrocene unit can be regarded as a side chain. However, this structure is not limited to polymers with an organic backbone , so that inorganic polymers with ferrocene side chains are also known and have interesting properties.
history
Shortly after the discovery of ferrocene in 1951, attempts were made to use the synthetic and structural possibilities of the molecule for polymerization reactions. The high temperature resistance of ferrocene offered the opportunity to develop high temperature resistant polymers, the redox properties seemed to allow use as a catalyst . Furthermore, there was technological potential in areas such as radiation protection as UV absorbers or as organic semiconductors .
The first synthesis of a polyferrocene was successful in 1955. In the 1960s, poly (1,1'-ferrocene) s were synthesized in good yields by coupling 1,1'-diiodoferrocene with magnesium . The condensation of ferrocene with aldehydes succeeded in 1963.
In 1992 Thomas B. Rauchfuss reported on the preparation of polyferrocenes by ring-opening polymerisation of ferrocenophanes . This method was widely used in the preparation of polyferrocenes; the polymers obtained have high molar masses that can be further processed into films. Apart from the deployed foot of flue persulfide bridges a wide variety of organic and inorganic bridges is suitable in Ferrocenophenen showing the polyferrocenes.
In 2016 a group from Imperial College London reported the preparation of oligomeric ferrocene rings with ring sizes between 5 and 9 ferrocene units.
Manufacturing
Ferrocene behaves like a conventional aromatic system in many reactions due to the aromaticity of the cyclopentadienyl ligands. An important reaction for the functionalization of ferrocene is the electrophilic aromatic substitution , whereby the presence of two rings allows an easier multiple substitution . By modifying the ferrocene unit with further substituents, such as alkyl chains , the solution properties of the polymer can be varied, for example.
Poly (1,1'-ferrocene) e
Poly (1,1'-ferrocene) s are made by various syntheses. The first synthesis was based on the action of di-tert-butyl peroxide on ferrocene. The products with a molar mass of about 7000 g / mol contained not only polymetallocenes, but also alkyl or ether-bridged units. The radical representation via dilithium ferrocenes also did not provide uniform products with sufficient chain length.
Poly (1,1'-ferrocenene-alkylene) e
Similarly, the reaction of the phenolic resins can be Methylcarbinolferrocen with from formaldehyde and dimethylamine prepared N , N -Dimethylaminomethylferrocen in the presence of zinc chloride and hydrogen chloride to methylene bridged poly (1,1'-ferrocene-methylene) en polymerize. However, the polymers obtained are only of a relatively short chain length.
The production proceeds better via ring-opening polymerisation of ferrocenophanes, also called ansa- ferrocenes. An ethylene-bridged ferrocenophane provides about poly (1,1'-ferrocen-ethylene) en. The driving force behind the polymerization is the ring strain , which increases as the tilt angle of the bridged cyclopentadienyl units increases.
Poly (1,1'-ferrocene-arylene) e
The first production of the poly (1,1'-ferrocene-arylene) s took place via poly recombination , in which the chain growth takes place via the initial initiator breakdown with transfer of the radical function to the oligomer and subsequent recombination. However, the polymers obtained in this way had only short chain lengths. The polymerization was more successful via the preparation of a 1,8-dicylopentadienylnaphthalene, which after conversion into the dianion could be polymerized with iron (II) chloride .
Poly (1,1'-ferrocene-silane) s and other heteroatom-bridged polyferrocenes
The production can take place via various methods such as the ring-opening polymerization of ferrocenophanes. The ferrocenophanes can be obtained by reacting dilithium ferrocene with, for example, dimethyldichlorosilane .
Poly (1,1'-ferrocene-phosphine) s can be obtained by polycondensation of 1,1'-dilithium ferrocene with dichlorophenylphosphine . The poly (1,1'-ferrocene-phosphane) s obtained in this way are temperature-resistant up to 350 ° C. Phosphorus bridged ferrocenophanes can be polymerized to homo- or mixed block copolymers by thermally or anionically induced ring-opening polymerization. As a result, poly (1,1'-ferrocene-phosphane-sulfide) s are accessible.
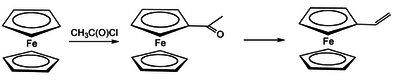
Poly (1-ferrocene-alkene) e
An important starting compound for the production of poly (1-ferrocene-alkene) s is vinyl ferrocene . This can be synthesized by reducing acetylcyclopentadienyl-cyclopentadienyl iron using lithium aluminum hydride via a methyl carbinol intermediate, which is thermally degraded to the vinyl derivative of ferrocene. The vinyl ferrocene can be easily homo- or copolymerized with other vinyl monomers such as styrene or methyl methacrylate .
Applications
The polyferrocenes are scientifically used as highly refractive polymers, for example as anti-reflective coatings or in light emitting diodes . Polyferrocenes are highly conductive after doping with iodine . Furthermore, ferrocene can be reversibly oxidized to ferrocinium ion. However, all of the applications mentioned are still in the experimental stage and have not yet found their way into technology despite promising approaches.
Coatings
When bombarded with satellites they are charged with charged particles from the solar wind . The charging can lead to an arc discharge , which can significantly impair the function of the satellite due to magnetic interference and material failure. To avoid these impairments, coatings of the electrically weak or non-conductive plastic components with thin films of poly (1,1'-ferrocene-silane) s were investigated. These divert the charge generated by the irradiation and could thus protect the satellite from rollovers.
Polymers with a high refractive index
Poly (1,1'-ferrocene-silane) s, poly (1,1'-ferrocene-phosphane) s and polyferrocenes with phenyl side chains are polymers with an unusually high refractive index , with values in the refractive index of up to 1.74 being achieved . The polyferrocenes have good film-forming properties and are considered candidates for photonic components.
Plasma-assisted reactive ion etching
Poly (ferrocene-dimethylsilane) s (PFS) were used as a barrier material in plasma-assisted reactive ion etching . Due to the presence of iron and silicon in the main chain, the polymer proved to be relatively stable compared to purely organic polymers. During the etching, a thin oxide layer containing iron and silicon was formed on the surface of the poly (ferrocene-dimethylsilane).
literature
- Ian Manners: Polymers and the Periodic Table: Recent Developments in Inorganic Polymer Science. In: Angewandte Chemie International Edition in English. 35, 1996, p. 1602, doi : 10.1002 / anie.199616021 .
- Jürgen Falbe, Manfred Regitz (Ed.): Römpp-Lexikon Chemie , 9th edition, Vol. 5, PI-S, 1999, ISBN 3-13-735010-7 , pp. 3449-3455.
Web links
Individual evidence
- ↑ a b c d e f g Jürgen Falbe, Manfred Regitz (ed.): Römpp-Lexikon Chemie , 9th edition, Vol. 5, PI-S, 1999, ISBN 3-13-735010-7 , p. 3449 -3455.
- ↑ Dennis C. Van Landuyt, Samuel F. Reed: Polymerization studies on 1-ferrocenyl-1,3-butadiene. In: Journal of Polymer Science Part A-1: Polymer Chemistry. 9, p. 523, doi : 10.1002 / pol.1971.150090224 .
- ↑ Rudolf Pietschnig: polymer with pendant Ferrocenes. In: Chemical Society Reviews 45, 2016, p. 5216, doi : 10.1039 / C6CS00196C .
- ^ TJ Kealy, PL Pauson: A New Type of Organo-Iron Compound. In: Nature. 168, 1951, p. 1039, doi : 10.1038 / 1681039b0 .
- ^ Eberhard W. Neuse, Harold Rosenberg: Metallocene Polymers. In: Journal of Macromolecular Science, Part C. 4, 2007, p. 1, doi : 10.1080 / 15321797008080022 .
- ↑ a b F. S. Arimoto, AC Haven: Derivatives of Dicyclopentadienyliron. In: Journal of the American Chemical Society. 77, 1955, p. 6295, doi : 10.1021 / ja01628a068 .
- ↑ Eberhard W. Neuse: Ferrocene-containing Polymers: Polycondensation of Ferrocene with Aldehydes. In: Nature. 204, 1964, p. 179, doi : 10.1038 / 204179a0 .
- ^ Paul F. Brandt, Thomas B. Rauchfuss: Polyferrocenylene persulfides. In: Journal of the American Chemical Society. 114, 1992, p. 1926, doi : 10.1021 / ja00031a083 .
- ↑ Michael S. Inkpen, Stefan Scheerer, Michael Linseis, Andrew JP White, Rainer F. Winter, Tim Albrecht, Nicholas J. Long: Oligomeric ferrocene rings. In: Nature Chemistry. 8, 2016, pp. 825-830, doi : 10.1038 / nchem.2553 .
- ^ A b Eberhard W. Neuse, Ronald K. Crossland: Metallocene polymers XIX. Polyferrocenylenes. In: Journal of Organometallic Chemistry. 7, 1967, p. 344, doi : 10.1016 / S0022-328X (00) 91087-8 .
- ^ Ian Manners: Polymers and the Periodic Table: Recent Developments in Inorganic Polymer Science. In: Angewandte Chemie International Edition in English. 35, 1996, p. 1602, doi : 10.1002 / anie.199616021 .
- ^ Harry R. Allcock: Inorganic-Organic Polymers. In: Advanced Materials. 6, 1994, p. 106, doi : 10.1002 / adma.19940060203 .
- ^ Ian Manners: Ring-opening polymerization of metallocenophanes. In: Advanced Materials. 6, 1994, p. 68, doi : 10.1002 / adma.19940060115 .
- ^ Ian Manners: Polyferrocenylsilanes: metallopolymers for electronic and photonic applications. In: Journal of Optics A: Pure and Applied Optics. 4, 2002, p. S221, doi : 10.1088 / 1464-4258 / 4/6/356 .
- ↑ a b R. Resende, A. Berenbaum, G. Stojevic, F. Jäkle, A. Bartole, F. Zamanian, I. Manners: Application of ring-opened poly (ferrocene) s as protective coatings for charge dissipation dielectrics . In: Advanced Materials , 12 (5), (2000), pp. 327-330.
- ^ Vasilios Bellas, Matthias Rehahn: Polyferrocenylsilane-Based Polymer Systems. In: Angewandte Chemie International Edition. 46, 2007, p. 5082, doi : 10.1002 / anie.200604420 .
- ↑ Rob GH Lammertink, Mark A. Hempenius, Vanessa Z.-H. Chan, Edwin L. Thomas, G. Julius Vancso: Poly (ferrocenyldimethylsilanes) for Reactive Ion Etch Barrier Applications. In: Chemistry of Materials. 13, 2001, p. 429, doi : 10.1021 / cm001052q .