Propane-1,3-sultone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
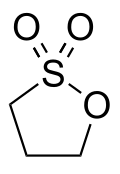
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Propane-1,3-sultone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 6 O 3 S | ||||||||||||||||||
| Brief description |
colorless, crystalline mass |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 122.14 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
31 ° C |
||||||||||||||||||
| boiling point |
96 ° C (1.3 hPa) |
||||||||||||||||||
| Vapor pressure |
0.48 Pa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Authorization procedure under REACH |
particularly worrying : carcinogenic ( CMR ) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Propane-1,3-sultone is a chemical compound from the substance group of sultones . It can be seen as an inner, cyclic ester of γ-hydroxypropanesulfonic acid .
Presentation and extraction
The compound is synthesized in two steps. In the first step, a radical addition of sodium hydrogen sulfite to allyl alcohol in the presence of atmospheric oxygen or peroxides as initiators produces sodium 3-hydroxypropanesulfonate. The target compound is obtained in the second step by acidic dehydration of the intermediate compound. Another synthesis starts with a Reed reaction with n- propyl chloride , chlorine and sulfur dioxide . The resulting γ-chloropropanesulfonic acid chloride is hydrolyzed to the free acid and then cyclized with the release of hydrogen chloride .
properties
Propane-1,3-sulton is a colorless, crystalline mass that melts at 31 ° C. The compound can only be vaporized under reduced pressure. The substance decomposes in the heat.
| Boiling temperatures under reduced pressure | ||||||||||||
| pressure | in mbar | 1.33 | 6.67 | 13.32 | 40 | |||||||
| Boiling temperature | in ° C | 100 | 134 | 150 | 180 | |||||||
The compound is only slightly soluble in water. At 25 ° C 0.025 mol% and at 70 ° C 0.062 mol% dissolve in water. Conversely, at 25 ° C., 0.75 mol% and at 70 ° C. 0.475 mol% of water dissolve in propane-1,3-sultone. A slow hydrolysis takes place in water, which accelerates with increasing temperature. The product of the reaction with water is 3-hydroxypropanesulfonic acid.
If there is an excess of water, the hydrolysis reaction can be seen as a first-order reaction , the half -lives of which have been determined in relation to the propane-1,3-sultone concentration.
| Hydrolysis rate | ||||||||||||
| temperature | in ° C | 20th | 30th | 40 | 70 | |||||||
| Half-life | in h | 14.8 | 4.8 | 1.6 | 0.11 | |||||||
The reaction of the compound with alcohols or alcoholates gives the corresponding 3-alkoxypropanesulfonic acids. Propane-1,3-sultone acts as an alkylating agent against many substances. The O- or S-alkylated products are formed with urea or thiourea . 3-sulfopropyl esters result with carboxylic acids . In the presence of aluminum chloride , the Friedel-Crafts alkylation of aromatics is successful .
use
The compound can be used for the production of polyether sulfones and special, zwitterionic polymers. It is also a component of battery electrolytes and photographic materials. In organic chemistry, it is used as the starting compound for the synthesis of sultaines , sulfobetaines , heterocycles and other sulfonyl-functionalized compounds.
Health hazards
Propane-1,3-sultone is classified as a particularly dangerous carcinogenic substance according to REACH and may only be manufactured or used in closed plants.
Individual evidence
- ↑ a b c d e f g h i j Entry on propane-1,3-sultone. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ a b c d e f Entry on 1,3-propane sultone in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c d e f g h i j k l m Fischer, RF: Propanesultone . In: Ind. Eng. Chem. 56 (1964) 41-45, doi : 10.1021 / ie50651a008 .
- ↑ Entry on 1,3-propanesultone in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet 1,3-propane sultone from Sigma-Aldrich , accessed on January 25, 2020 ( PDF ).
- ↑ a b Entry in the SVHC list of the European Chemicals Agency , accessed on August 19, 2016.
- ↑ a b Bordwell, FG; Osborne, E .; Chapman, D .: The Hydrolysis of Sultones. The Effect of Methyl Groups on the Rates of Ring-opening Solvolyses in J. Am. Chem. Soc. 81 (1959) 2698-2705, doi : 10.1021 / ja01520a029 .



