Silver (I) oxide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Ag + __ O 2− | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Silver (I) oxide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | Ag 2 O | ||||||||||||||||||
| Brief description |
heavy, almost black, velvety powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 231.74 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
7.2 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
130 ° C (decomposition) |
||||||||||||||||||
| solubility |
practically insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
0.01 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−31.1 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Silver (I) oxide (Ag 2 O) is a chemical compound from the group of oxides .
Extraction and presentation
Silver (I) oxide is the reaction product of the noble metal silver with oxygen .
Sodium hydroxide or potassium hydroxide is added to the silver nitrate solution . Silver oxide precipitates in alkaline as a brown precipitate :
properties
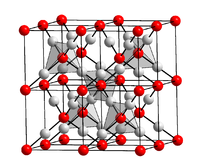
Silver (I) oxide is a brown powder that darkens when exposed to sunlight. Moist silver (I) oxide is very insensitive to light and decomposes somewhat when it dries. It has a crystal structure of the Cu 2 O type with the space group Pn 3 m (space group number 224) (a = 475.2 pm) and an enthalpy of formation of −30.5 kJ / mol. Slurries of silver oxide in water have a markedly alkaline reaction, since silver and hydroxide ions are formed in reverse of the above reaction.
In reverse of the synthesis reaction, silver (I) oxide is broken down again into the elements silver and oxygen when heated ( thermolysis ).
In the air, silver (I) oxide reacts with carbon dioxide to form silver carbonate :
use
In preparative organic chemistry, silver (I) oxide is used in a variant of the Williamson ether synthesis .
Silver (I) oxide is contained in thermal paste to conduct the processor heat to the heat sinks in the computer, as it has a high thermal conductivity .
Silver (I) oxide is part of the silver oxide-zinc battery that is used in wristwatches and other small devices.
Individual evidence
- ↑ Entry on SILVER OXIDE in the CosIng database of the EU Commission, accessed on May 4, 2020.
- ↑ Entry on silver oxides. In: Römpp Online . Georg Thieme Verlag, accessed on September 19, 2014.
- ↑ a b c d e f g h Entry on silver (I) oxide in the GESTIS substance database of the IFA , accessed on November 18, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-4.
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 998.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ Masato Tanabe and Richard H. Peters: (R, S) -Mevalonolactone-2- 13 C In: Organic Syntheses . 60, 1981, p. 92, doi : 10.15227 / orgsyn.060.0092 ; Coll. Vol. 7, 1990, p. 386 ( PDF ).







