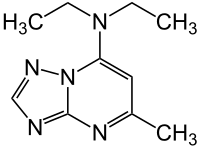
Trapidil
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Trapidil | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 10 H 15 N 5 | |||||||||||||||||||||
| Brief description |
white to almost white, crystalline powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 205.26 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
98-99 ° C |
|||||||||||||||||||||
| pK s value |
2.79 |
|||||||||||||||||||||
| solubility |
Easily soluble in water, soluble in dichloromethane and anhydrous ethanol |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Trapidil is a drug that is used in the form of capsules and injections for the treatment of coronary artery disease (CHD), for the prophylaxis of cardiovascular complications after myocardial infarction and in the therapy of peripheral arterial disease . It acts primarily as a non-selective phosphodiesterase inhibitor by widening the coronary vessels and also inhibits platelet aggregation . Trapidil was developed in the 1960s by the hydrogenation plant in Rodleben and at the Institute for Pharmacology at the Martin Luther University in Halle-Wittenberg .
Clinical information
Application areas (indications)
Trapidil is primarily used for the treatment of coronary artery disease (CHD) by reducing the preload and afterload of the heart muscle , especially in patients with an intolerance to the standard active ingredients from the nitrate class . It also reduces the incidence of other cardiovascular complications after a myocardial infarction . In patients with peripheral arterial disease , Trapidil improves blood flow to the limbs and improves walking ability.
type of application
Trapidil is used either orally in capsule form or by intravenous injection . The use in drug-eluting stents ( drug-eluting stents ) is in clinical testing .
Adverse effects (side effects)
Uncommon side effects of treatment with Trapidil are nausea and vomiting , loss of appetite , abdominal discomfort and bloating, and headache . The uncommon undesirable effects include allergic reactions , orthostatic hypotension and a feeling of pressure in the chest area , both of which may be associated with too rapid intravenous administration. Serious complications have not yet been described.
Pharmacological properties
Mechanism of action (pharmacodynamics)
Trapidil mainly acts as a non-selective phosphodiesterase inhibitor by widening the coronary vessels ( vasodilation ). In addition, it inhibits platelet aggregation and the synthesis of thromboxane A2 , which increases the flowability of the blood . It also regulates calcium levels in vascular smooth muscle cells and heart muscle cells .
Uptake and elimination (pharmacokinetics)
Trapidil has a high bioavailability when ingested and a half-life of around 2.4 hours. The biotransformation takes place primarily in the liver , the most important metabolite is desethyltrapidil. Repeated use is likely to induce the corresponding metabolizing enzymes . The excretion takes place within 24 hours, almost entirely on the kidney .
Other Information
History
Trapidil was synthesized for the first time in 1964 at the VEB Deutsches Hydrierwerk in Rodleben in what was then the German Democratic Republic (GDR), examined in the following years at the Institute of Pharmacology at the Martin Luther University Halle-Wittenberg and approved for the GDR market in 1971. Three years later, a license was granted for distribution in Japan , where it has been approved since 1979 and is the most widely prescribed agent for the treatment of CHD. In addition, it is also relevant in the Italian pharmaceutical market. It is one of the few drugs from drug research in the GDR that has also gained importance in western countries. The approval in the Federal Republic of Germany took place in 1992 under the trade name Rocornal .
Trade names
Rocornal ( D , A , CZ , JP ), Avantrin ( I ), Travisco ( BR ), Trapidil ( INN )
literature
- Jürgen Stoschek: Coronary therapeutic agent Trapidil: New facets of a classic. In: Deutsches Ärzteblatt . 97 (37) / 2000. A-2406 / B-2057 / C-1928.
- Mazhar Khan, Fiona Ní Mhulláin, Margaret Nolan: Intrepide; Trapidil Eluting Stent. In: EuroIntervention. 4 (3) / 2008. Europa Edition, pp. 405-411, PMID 19110816 .
- Trapidil. In: Arthur Asao Sasahara, Joseph Loscalzo: New Therapeutic Agents in Thrombosis and Thrombolysis. Series: Fundamental and Clinical Cardiology. Volume 46. Informa Health Care, Hoboken 2003, ISBN 0-8247-0795-8 , pp. 464/465.
- Trapidil. In: Berndt Lüderitz: 75 Years of the German Society for Cardiology - Heart and Circulatory Research. Springer, Berlin 2002, ISBN 3-540-41431-2 , pp. 112/113 (historical information).
Individual evidence
- ↑ a b TRAPIDIL CRS data sheet (PDF) at EDQM , accessed on December 26, 2009.
- ↑ a b c d Entry on Trapidil. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b Data sheet Trapidil, ≥98% (HPLC) from Sigma-Aldrich , accessed on November 1, 2016 ( PDF ).