α- N -acetylgalactosaminidase
| α- N -acetylgalactosaminidase | ||
|---|---|---|

|
||
| Ribbon model of the human α- N -acetylgalactosaminidase, according to PDB 3H53 . | ||
| other names |
|
|
|
Existing structural data : 1KTB , 1KTC , 3H53 , 3H54 , 3H55 , 3IGU , 5WZN , 5WZP , 5WZQ , 2IXB , 5WZR |
||
| Properties of human protein | ||
| Mass / length primary structure | 411 amino acids | |
| Secondary to quaternary structure | Homodimer | |
| Identifier | ||
| Gene names | NAGA ; GALB; D22S674 | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 3.2.1.49 , hydrolase | |
| Response type | hydrolysis | |
| Substrate | terminal α- N -acetylgalactosamine residues | |
| Products | N -acetylgalactosamine + aglycon | |
| Occurrence | ||
| Homology family | HOG000161224 | |
| Parent taxon | Bacteria , eukaryotes | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 4668 | 17939 |
| Ensemble | ENSG00000198951 | ENSMUSG00000022453 |
| UniProt | P17050 | Q9QWR8 |
| Refseq (mRNA) | NM_000262 | NM_008669 |
| Refseq (protein) | NP_000253 | NP_032695 |
| Gene locus | Chr 22: 42.06 - 42.07 Mb | Chr 15: 82.33 - 82.34 Mb |
| PubMed search | 4668 |
17939
|
The α- N -acetylgalactosaminidase (short: Nagalase , EC 3.2.1.49 )) is an enzyme that belongs to the glycosidases . Nagalase reversibly catalyzes the cleavage of N -acetylgalactosamine from larger molecules ( glycoconjugates ). In humans, the NAGA gene codes for the Nagalase.
Mutations in NAGA are very rare and lead to an α- N -acetylgalactosaminidase deficiency with a corresponding clinical picture: Schindler's disease and, in the adult form, Kanzaki's disease. It was postulated that Nagalase is secreted by cancer cells, which is supposed to facilitate the proliferation of cancer cells by weakening the immune system. The relevant scientific publications have been revoked by some journals due to significant quality defects.
Various conspiracy theories are spread by opponents of vaccinations , for example Nagalase is said to be contained in vaccines . This is not the case.
Bacterial Nagalases have been proposed for biotechnological application: Red blood cells of blood group A can be converted to blood group 0 by enzymatic splitting off of N- acetylgalactosamine . In the meantime, however, another, much more efficient system with two enzymes is being discussed.
structure
The human Nagalase is composed of two domains. Domain 1 contains eight α / β-barrels and domain 2 contains eight anti-parallel β-strands in two β-sheets . Nagalase is also a highly glycosylated and disulfide-rich glycoprotein . The mature wild-type protein contains five N- linked glycosylation sites ( Asn124 , Asn177, Asn201, Asn359 and Asn385), four disulfide bridges ( Cys38 -Cys80, Cys42-Cys49, Cys127-Cys158 and Cys343) (Cys343) (Cys343).
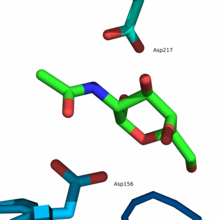
The active center is located in the α / β-barrel at the C -terminal end of the β-strands of domain 1. The active center is formed by loops that are C -terminal to the six consecutive β-strands (β1-β6) . Amino acid residues that make up the active site include Trp33 , Asp78 , Asp79, Tyr119 , Cys127, Lys154 , Asp156, Cys158, Ser188 , Ala191 , Tyr192, Arg213, and Asp217. In addition, Tyr192 and the disulfide bridge Cys127-Cys158 ensure that an α-anomer is selected as substrate .
function
Conversion of blood group A to blood group 0
The AB0 system is based on the presence or absence of blood group antigens A and B. The antigens consist of carbohydrate structures that are located at the ends of oligosaccharide chains of glycoproteins or glycolipids . The glycoproteins or glycolipids are found on the surface of erythrocytes, as well as endothelial and most epithelial cells . The immunodominant monosaccharide , which determines the specificity of blood group A, is an α-1,3-bound N- acetylgalactosamine, which is terminally bound to an oligosaccharide chain. In addition, antibodies are always formed against the non-existent antigens, with blood group A that is antibodies against B and vice versa, with blood group AB no antibodies and with blood group 0 antibodies against A and B. Therefore, individuals with anti-A (blood group B) - and / or anti-B (blood group A) antibodies do not receive a blood transfusion with incompatible antigens, as this would otherwise activate the complement system and ultimately dissolve the erythrocytes, which often cause an acute hemolytic transfusion reaction (AHTR) or other reactions. The erythrocytes of blood group 0 contain neither A nor B antigens, which is why the blood transfusion of individuals with blood group 0 can be carried out in carriers of all other blood groups.
The immunodominant trisaccharide - epitope of the antigen A ( 1 ) is obtained from the disaccharide epitope ( 2 using) the enzyme α-1,3- N -Acetylgalactosaminyltransferase formed (GTA), wherein the disaccharide epitope in the antigen H (blood group 0) occurs. A bacterial exoglycosidase is used to convert blood group A to blood group 0 , for example the Nagalase of the bacterium Elizabethkingia meningoseptica , which hydrolyzes the glycosidic bond and thus catalyzes the reverse reaction. Because of its ability to convert blood groups from A to 0, Nagalase is also known under the name A-zym .
mechanism
The reaction mechanism at the active center follows the so-called ping-pong mechanism , whereby the α-anomeric substrate is cleaved by two successive nucleophilic attacks on the anomeric center and an α-anomer is also obtained as the product. Two carboxylate groups are required to split the glycosidic bond , one acting as a nucleophile and the other as an acid and then as a base . The first step is the nucleophilic attack of the carboxylate group of Asp156 on the C1 atom of the substrate, which leads to the cleavage of the glycosidic bond and the release of the aglycone (R – OH), which is then protonated by Asp217 . The resulting formation of a high-energy covalent intermediate in a twist conformation leads to the subsequent hydrolysis of the intermediate by a water molecule , which was previously deprotonated by Asp217 and thereby generates a hydroxide ion . The hydroxide ion carries out a nucleophilic attack on the C1 atom of the covalent intermediate, which leads to the cleavage of Asp156 and the formation of N- acetylgalactosamine as the end product.
Web links
- Questions and answers about the Nagalase test. In: nagalase-test.de. Retrieved January 15, 2020 .
Individual evidence
- ↑ NAGA alpha-N-acetylgalactosaminidase [Homo sapiens (human)] genes - NCBI. Retrieved January 15, 2020 .
- ↑ a b Kathrin Helmreich: "All doctors who found cancer-causing enzymes in vaccines are dead!" In: mimikama . January 11, 2018, accessed January 15, 2020 .
- ↑ a b Ralf Nowotny: "Cancer Enzyme" in Vaccines: Did Doctors Have to Die for That? (Fact check). In: mimikama . January 9, 2020, accessed January 15, 2020 .
- ↑ a b Qiyong P. Liu et al .: Bacterial glycosidases for the production of universal red blood cells . In: Nature Biotechnology . tape 25 , no. 4 , April 2007, pp. 454-464 , doi : 10.1038 / nbt1298 , PMID 17401360 .
- ↑ Peter Rahfeld and Stephen G. Withers: Toward universal donor blood: type Enzymatic conversion of A and B to O . In: The Journal of Biological Chemistry . tape 295 , no. 2 , January 10, 2020, p. 325–334 , doi : 10.1074 / jbc.REV119.008164 , PMID 31792054 , PMC 6956546 (free full text).
- ↑ a b N. E. Clark, SC Garman: The 1.9 a structure of human alpha-N-acetylgalactosaminidase: The molecular basis of Schindler and Kanzaki diseases. In: Journal of molecular biology. Volume 393, number 2, October 2009, pp. 435-447, doi : 10.1016 / j.jmb.2009.08.021 , PMID 19683538 , PMC 2771859 (free full text).
- ^ WM Watkins: Biochemistry and Genetics of the ABO, Lewis, and P blood group systems. In: Advances in human genetics. Volume 10, 1980, pp. 1-136, 379, doi : 10.1007 / 978-1-4615-8288-5_1 , PMID 6156588 (review).
- ↑ H. Clausen, S. Hakomori: ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. In: Vox sanguinis. Volume 56, Number 1, 1989, pp. 1-20, doi : 10.1111 / j.1423-0410.1989.tb03040.x , PMID 2464874 (Review).
- ↑ K. Sazama: Transfusion errors: scope of the problem, consequences, and solutions. In: Current hematology reports. Volume 2, Number 6, November 2003, pp. 518-521, PMID 14561397 (review).
- ↑ D. Stainsby, H. Jones, D. Asher, C. Atterbury, A. Boncinelli, L. Brant, CE Chapman, K. Davison, R. Gerrard, A. Gray, S. Knowles, EM Love, C. Milkins , DB McClelland, DR Norfolk, K. Soldan, C. Taylor, J. Revill, LM Williamson, H. Cohen: Serious hazards of transfusion: a decade of hemovigilance in the UK. In: Transfusion medicine reviews. Volume 20, number 4, October 2006, pp. 273-282, doi : 10.1016 / j.tmrv.2006.05.002 , PMID 17008165 .