1-octen-3-ol
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| Structure of ( R ) - (-) - 1-octen-3-ol | ||||||||||
| General | ||||||||||
| Surname | 1-octen-3-ol | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 8 H 16 O | |||||||||
| Brief description |
colorless liquid with a characteristic odor |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 128.22 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
0.835 g cm −3 |
|||||||||
| Melting point |
−49 ° C |
|||||||||
| boiling point |
175 ° C |
|||||||||
| Vapor pressure |
1 hPa (20 ° C) |
|||||||||
| solubility |
poorly soluble in water |
|||||||||
| Refractive index |
1.4391 (12 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
1-Octen-3-ol ( Octenol for short ) is a chemical compound . In its pure state it is a colorless liquid with a musty odor, which is difficult to ignite and can form explosive mixtures with air. It belongs to the alcohols , especially to the allyl alcohols , and is widespread in nature as a metabolic product.
The taste of octenol can still be felt in food down to the ppb range.
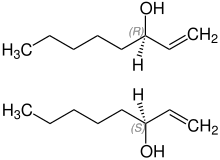
Stereochemistry
Octenol is a chiral compound that contains an asymmetrically substituted carbon atom. The synthetically produced compound is therefore usually a 1: 1 mixture ( racemate ) of two mirror-image molecules ( enantiomers ):
- ( R ) - (-) - 1-octen-3-ol and
- ( S ) - (+) - 1-octen-3-ol
These differ in the rotation value and their physiological effects. The individual stereoisomers can be obtained in a targeted manner using suitable synthesis strategies or separation processes.
Occurrence
Octenol is the taste-determining substance in many edible mushrooms. In particular, it arises at the moment of cutting the mushroom meat.
It is the main ingredient in the essential oil of rhododendron and determines the taste of the potato tuber . Wild bergamot , oregano and West Indian laurel still contain it in no small quantities . It counteracts mold infestation such as Penicillium or Aspergillus and is probably produced as a secondary substance by fungi and plants for this reason.
Octenol is a metabolic product of many microorganisms such as the Penicillium mold . It is one of the substances that cause the musty smell of moldy apartments and remains noticeable for a long time after the mold has been combated. Moldy vegetables and old books also owe their aroma in part to the octenol.
Octenol is partly responsible for many food flavors such as B. the smell and taste of blue cheese and camembert or the cork tone of the wine .
Role in insect metabolism
Along with 4-hydroxy-4-methyl-2-pentanone , 1-hepten-3-one and 3-octanone, octenol is one of those odor-intensive substances that, coming from plants and fungi, attract female insects and induce them to lay their eggs. 40 percent of the olfactory cells of the tsetse fly respond to octenol.
Manufacturing and Biosynthesis
Chemically, octenol can be produced in two ways:
- by Grignard reaction of acrolein with amyl iodide in 65 percent yield
- by selective reduction of 1-octen-3-one in 90 percent yield
Biochemically, ( R ) - (-) - octenol is formed by peroxidation of linoleic acid , catalyzed by a lipoxygenase , and subsequent cleavage of the hydroperoxide with the help of a hydroperoxide lyase . This reaction also takes place in cheese and is used biotechnologically to produce the ( R ) - (-) - enantiomer.
use
Octenol is used as an attractant for tsetse flies , mosquitoes and mosquitoes in professional attractant traps . Of the two stereoisomers , only ( R ) - (-) - 1-octen-3-ol is effective here. Usually, however, the 1: 1 mixture ( racemate ), which is technically easier to synthesize, is used.
It is also used as a perfume ingredient. Some perfumes contain up to 1 percent.
hazards
Octenol vapors were classified as non-toxic; for ingested octenol there is an LD 50 of 340 mg / kg and an LD 50 of 3300 mg / kg on the skin . It is considered to be irritating to the eyes in large quantities.
Inamdar and co-workers found a disruption of the dopamine balance due to 1-octen-3-ol in Drosophila and in human cell lines . This calls into question the non-toxicity of octenol vapors. Octenol vapors in moldy rooms could be responsible for neuropsychological problems and movement disorders. Octenol could be a contributing factor to Parkinson's disease .
Individual evidence
- ↑ Entry on AMYLVINYL CARBINOL in the CosIng database of the EU Commission, accessed on February 25, 2020.
- ↑ a b c d e f g h i j k Entry for CAS no. 3391-86-4 in the GESTIS substance database of the IFA , accessed on April 20, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-404.
- ↑ MJ Saxby: Food taints and off-flavors. , 2nd edition, Springer 1995 , ISBN 978-0-7514-0263-6 , pp. 210-212.
- ↑ DO Okull: Antifungal activity of 10-oxo-trans-8-decenoic acid and 1-octen-3-ol against Penicillium expansum in potato dextrose agar medium. J. Food Prot. 66/8/ 2003 . Pp. 1503-1505; PMID 12929847 .
- ↑ L. Moio et al .: Odor-impact compounds of Gorgonzola cheese. J. Dairy Res. 67/2/ 2000 . Pp. 273-285; PMID 10840681 .
- ↑ O. Ezquerro O and MT Tena: Determination of odor-causing volatile organic compounds in cork stoppers by multiple headspace solid-phase microextraction. J. Chromatogr. A 1068/2/ 2005 . Pp. 201-208; PMID 15830925 .
- ↑ a b W. Schwab and P. Schreier: Enzymic Formation of Flavor Volatiles from Lipids. in HW Gardner and TM Kuo: Lipid Biotechnology. , CRC Press, 2002 , ISBN 0-8247-0619-6 .
- ↑ SP Gouinguené and E. Städler: Oviposition in Delia platura (Diptera, Anthomyiidae): the role of volatile and contact cues of bean. J. Chem. Ecol. 32/7/ 2006 p 1399-1413; PMID 16718565 .
- ↑ RB Barrozo and CR Lazzari: The response of the blood-sucking bug Triatoma infestans to carbon dioxide and other host odors. Chem. Senses. 29/4/ 2004 . Pp. 319-329; PMID 15150145 .
- ^ Z. Syed and PM Guerin: Tsetse flies are attracted to the invasive plant Lantana camara. J. Insect. Physiol. 50/1/ 2004 . Pp. 43-50; PMID 15037092 .
- ^ S. Wnuk et al .: Synthesis and analysis of 1-octen-3-ol, the main flavor component of mushrooms. Food 27/5/ 1983 p 479-486; PMID 6684212 .
- ↑ K. Matsui et al .: Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes. In: Bioscience, Biotechnology, and Biochemistry 67/10/ 2003 p 2280-2282; PMID 14586122 .
- ↑ WA Qualls and GR Mullen: Evaluation of the Mosquito Magnet Pro trap with and without 1-octen-3-ol for collecting Aedes albopictus and other urban mosquitoes. J Am Mosq Control Assoc 23/2/ 2007 pp 131-136; PMID 17847844 .
- ↑ a b U.S. Environmental Protection Agency: Octenol Fact Sheet: 1-Octen-3-ol (069037) & R - (-) - 1-Octen-3-ol (069038) Fact Sheet. 07/05/07 .
- ^ AA Inamdar, MM Hossain et al. a .: Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration. In: Proceedings of the National Academy of Sciences. October 8, 2013, doi: 10.1073 / pnas.1318830110 .