Cyclam
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclam | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 24 N 4 | |||||||||||||||
| Brief description |
white to beige crystal powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 200.32 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point | ||||||||||||||||
| solubility |
soluble in water (50 g l −1 at 20 ° C ), soluble in methanol |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
As a so-called azamacrocyclus, cyclam is the nitrogen analogue of the crown ether 1,4,8,11-tetraoxa-cyclotetradecane (14-crown-4) and forms with divalent ions of transition metals , e.g. B. copper or nickel , stable complexes. The four secondary amino groups of the fourteen-membered [14] aneN 4 ring system form a tetradentate complex ligand, the metal chelates of which can exist in six different diastereomeric conformations.
Nickel-cyclam complexes are being investigated as catalysts for the light-induced photoreduction of carbon dioxide CO 2 to carbon monoxide CO. Cyclam complexes with radioactive isotopes of the transition elements as well as those of nitrogen-substituted cyclam are of interest as radiotherapeutic and radiodiagnostic agents .
Occurrence and representation
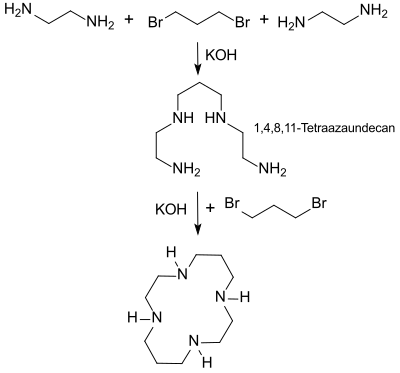
The representation of 1,4,8,11-tetraazacyclotetradecane was first published in 1936. In the first stage, 1,3-dibromopropane is reacted with an excess of 1,2-diaminoethane in ethanol in the presence of potassium carbonate , mainly N, N-bis (2-aminoethyl) -1,3-diaminopropane (1, 4,8,11-tetraazaundecane) is formed as a colorless liquid with a yield of 39% or 47%.
In later work, the tetraazaundecane is then reacted with further 1,3-dibromopropane under cyclization conditions (high dilution in ethanolic solution), the cyclam being obtained in a yield of 6%, i.e. H. the overall yield is only a maximum of 3% with 1,3-dibromopropane as the expensive starting material. As Hermann Stetter and co-workers were able to show, the direct reaction of 1,3-dibromopropane with ethylenediamine according to van Alphen produces only very small amounts of cyclam. The complex six-step synthesis according to Stetter using the tosyl protective group and under cyclization conditions produces cyclam in a total yield of approx. 20%.
A detailed procedure based on the Stetter process for the preparation of macrocyclic polyamines indicates an overall yield for the intermediate tetratosyl-cyclam of 77%.
In the period that followed, there were further attempts to increase the overall yield of cyclam and to reduce its price with cheaper preliminary products.
Replacing the tosyl groups with triflyl groups , which can be split off more gently with sodium amide in liquid ammonia , gives cyclam in 57% yield.
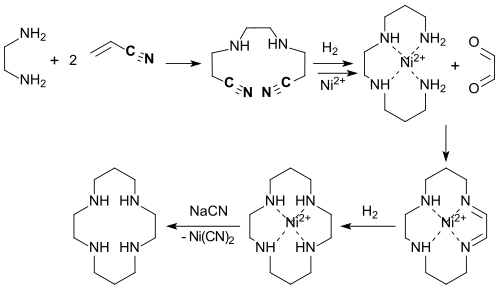
The observation that Ni 2+ ions interact strongly, especially with secondary polyamines, suggested that this would lead to a pre-orientation of the primary amino groups, which should facilitate ring closure to the cyclam. This kinetic “template effect ” of the Ni 2+ ion in the cyclization of 1,5,8,12-tetraazadodecane with glyoxal , subsequent hydrogenation and complexation of the Ni 2+ by heating with excess sodium cyanide (demetallization) actually increases the overall yield of cyclam approx. 20%.
In a more recent work on the template route by hydrogenation with a Raney nickel alloy in the cyclization with glyoxal and subsequent reactions, a yield of 67% is achieved, which is not achieved in the reduction of the intermediate diimino intermediate with sodium borohydride with 32% and 39% yield, respectively can be achieved.
The Michael addition of ethylenediamine to acrylonitrile (60% yield), the subsequent hydrogenation of the dicyano compound in ethanol with Raney nickel to give the primary diamine (60%), the cyclization of the nickel complex with glyoxal in water with renewed appear as an interesting alternative route with cheap starting materials Hydrogenation of the diimino intermediate and finally the demetallization of the Ni 2+ complex formed with sodium cyanide (60%).
With a total yield of 21% - albeit also using toxic cyanide - this synthesis appears considerably more economical.
An even stronger and more rigid pre-orientation of the amino groups accessible for the cyclization step is brought about by bis- aminal formation of the 1,4,8,11-tetraazaundecane precursor with 2,3-butanedione .
The cleavage of the bis-aminal with hydrochloric acid is considerably milder and more efficient than the cleavage of the tosyl groups by heating with concentrated sulfuric acid and gives the tetrahydrochloride of the cyclam in 77% overall yield.
properties
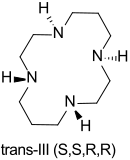
Cyclam is obtained as a white, hygroscopic and air-sensitive crystal powder or as needles that are soluble in water and methanol. The six possible conformations in metal complexes consist of a sequence of five- and six-membered chelate rings, the “trans-III” conformation being the most thermodynamically stable.
This in contrast to other cyclic tetramines that as hygroscopic and CO 2 are present absorbing oily liquids or low melting solids, crystalline 1,4,8,11-tetraazacyclotetradecane starts from 120 ° C to sublimate . It can be sublimed for purification or recrystallized from tetrahydrofuran or 1,4-dioxane . The pH value of a dilute aqueous cyclam solution (10 g · l −1 ) is 12.1.
Applications
The macrocyclic complex ligand 1,4,8,11-tetraazacyclotetradecane forms u. a. with divalent copper (logK = 27.2 and nickel ions (logK = 22.2) stable square-planar complexes that form yellow solutions in water and methanol.
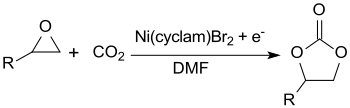
The Ni + (cyclam) complex formed during the electrochemical reduction of the nickel-cyclam complex Ni 2+ (cyclam) Br 2 catalyzes the conversion of epoxides with carbon dioxide to cyclic carbonates in high yields (91% for R = phenyl).
Because of their ability to catalyze the electrochemical reduction of carbon dioxide to carbon monoxide , nickel-cyclam complexes have been worked on in the past by several working groups, including that of Jean-Pierre Sauvage . The current interest in the utilization of CO 2 as a chemical raw material source could give new impetus to research on metal complexes of cyclam (derivatives) and other azamacrocycles.
The N-alkylation of cyclam is often not very productive, but can be achieved by converting alkyl and benzyl bromides in sodium hydroxide / acetonitrile mixture by simply shaking (not stirring!) Of cyclam with z. B. propargyl bromide can be brought up to 74% yield.
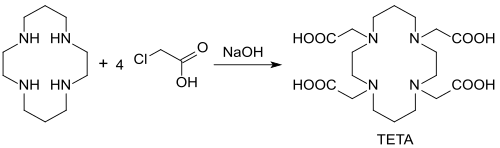
The complexing agent TETA (1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid) can be obtained from cyclam with monochloroacetic acid in an alkaline-aqueous medium with 69% yield.
TETA forms very stable complexes with the alkaline earth metals calcium and strontium and much less stable complexes with magnesium .
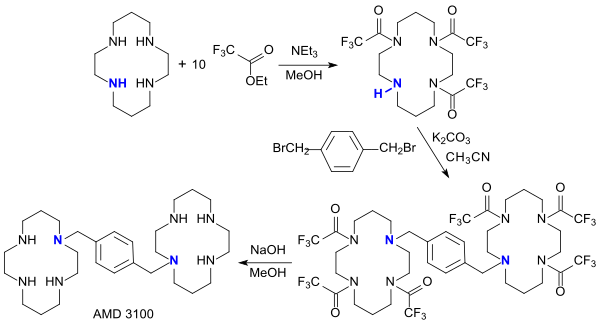
A more recent work describes an optimized synthesis for Plerixafor (AMD 3100, Mozobil TM ), originally developed as an HIV therapeutic agent and now marketed as an active ingredient for releasing stem cells .
Thereafter, in the reaction of cyclam with ethyl trifluoroacetate in a 10-fold excess, the trifluoroacetylated cyclam is selectively obtained, which reacts with 1,4-bis (bromomethyl) benzene (p-xylylene dibromide) in a total yield of 72% to form bis-cyclam AMD 3100 .
Functionalized cyclams have an increased binding capacity for strongly paramagnetic ions, such as. B. Gadolinium (III) complexes thereof as MRT - contrast agents find use for medical diagnostics. Stable complexes with radioactive isotopes of copper or technetium , such as. B. 64/67 Cu or 99m Tc can be covalently linked to antibodies and used for the specific control of tumor cells.
literature
- Luigi Fabbrizzi: A Lifetime Walk in the Realm of Cyclam in Macrocyclic and Supramolecular Chemistry: How Izatt-Christensen Award Winners Shaped the Field . Ed .: Reed M. Izatt. John Wiley & Sons, Chichester, UK 2016, ISBN 978-1-119-05384-2 , pp. 165-199 .
- Xiaoqi Yu, Ji Zhang: Macrocyclic Polyamines - Synthesis and Applications . Wiley-VCH, Weinheim 2017, ISBN 978-3-527-34187-0 .
Individual evidence
- ↑ a b c d Entry on 1,4,8,11-tetraazacyclotetradecane at TCI Europe, accessed on June 28, 2019.
- ↑ a b c data sheet 1,4,8,11-tetraazacyclotetradecane from Sigma-Aldrich , accessed on June 28, 2019 ( PDF ).
- ↑ a b c d data sheet 1,4,8,11-tetraazacyclotetradecane, 98% from AlfaAesar, accessed on June 28, 2019 ( PDF )(JavaScript required) .
- ^ B. Bosnich, ML Tobe, GA Webb: Complexes of nickel (II) with a cyclic tetradentate secondary amine . In: Inorg. Chem. Band 4 , no. 8 , 1965, p. 1109-1112 , doi : 10.1021 / ic50030a004 .
- ↑ JD Froehlich, CP Kubiak: Homogeneous CO 2 reduction by Ni (cyclam) at a glassy carbon electrode . In: Inorg. Chem. Band 51 , no. 7 , 2012, p. 3932-3934 , doi : 10.1021 / ic3001619 .
- ↑ a b c X. Liang, PJ Sadler: Cyclam complexes and their application in medicine . In: Chem. Soc. Rev. Band 33 , no. 4 , 2004, p. 246-266 , doi : 10.1039 / B313659K .
- ↑ Anika Röhrich: Synthesis and characterization of cyclam-based multimers as a basis for radiopharmaceuticals. (PDF; 1.5 MB) Technische Universität Dresden, 2009, accessed on June 28, 2019 .
- ^ J. van Alphen: On aliphatic polyamines III . In: Rec. Trav. Chim. Pays-Bas . tape 55 , no. 10 , 1936, pp. 835-840 , doi : 10.1002 / 19360551004 .
- ^ J. van Alphen: On aliphatic polyamines IV . In: Rec. Trav. Chim. Pays-Bas . tape 56 , no. 4 , 1937, pp. 343-350 , doi : 10.1002 / 19370560405 .
- ↑ a b Patent EP0427595A1 : Process for the synthesis of cyclic polynitrogenated compounds. Applied on October 31, 1990 , published on May 15, 1991 , applicant: L'Air Liquide SA, inventors: R. Guilard, I. Meunier, C. Jean, B. Boisselier-Cocolios.
- ↑ a b E.J. Bounsall, SR Koprich: Synthesis and characterization of cyclam complexes of rhodium (III) . In: Can. J. Chem. Volume 48 , no. 10 , 1970, p. 1481-1491 , doi : 10.1139 / v70-243 .
- ↑ a b H. Stetter, K.-H. Mayer: On the knowledge of the macrocyclic ring systems, VII, production and properties of macrocyclic tetramines . In: Chem. Ber. tape 94 , no. 6 , 1961, pp. 1410-1416 , doi : 10.1002 / cber.19610940602 .
- ↑ TJ Atkins, JE Richman, WF Oettle: 1,4,7,10,13,16-hexaazacyclooctadecane In: Organic Syntheses . 58, 1978, p. 86, doi : 10.15227 / orgsyn.058.0086 ; Coll. Vol. 6, 1988, p. 652 ( PDF ).
- ↑ V. Panetta, J.-J. Yaouane, H. Handel: Triflamides for protection and cyclization of tetraamines to tetraazamacrocycles . In: Tetrahedron Lett. tape 33 , no. 38 , 1992, pp. 5505-5508 , doi : 10.1016 / S0040-4039 (00) 61129-2 .
- ↑ a b K. Barefield: New synthesis of 1,4,8,11-tetraazacyclotetradecane (Cyclam) via the nickel (II) complex . In: Inorg. Chem. Band 11 , no. 9 , 1972, p. 2273-2274 , doi : 10.1021 / ic50115a065 .
- ↑ EK Barefield, F. Wagner, AW Herlinger, AR Dahl, S. Holt: (1,4,8,11-tetraazacyclotetradecane) nickel (II) perchlorate and 1,4,8,11-tetraazacyclotetradecane . In: Inorg. Synth. tape 16 , 1976, p. 220–225 , doi : 10.1002 / 9780470132470.ch58 .
- ^ I. Meunier, AK Mishra, B. Hanquet, P. Cocolios, R. Guilard: Synthesis and characterization of various unsubstituted and mono-N-substituted tetraazamacrocycles . In: Can. J. Chem. Volume 73 , no. 5 , 1995, p. 685-695 , doi : 10.1139 / v95-087 .
- ↑ DE Berry, S. Girard, A. McAuley: The synthesis and reactions of nickel (III) stabilized by a nitrogen-donor macrocycle . In: J. Chem. Educ. tape 73 , no. 6 , 1996, pp. 551-554 , doi : 10.1021 / ed073p551 .
- ↑ Radoslaw Marian Kierat: Synthesis, modification and biological application of fluorescent xanthine dyes. (PDF; 4 MB) Ruprecht-Karls-Universität Heidelberg, 2008, pp. 108-109 , accessed on July 20, 2019 .
- ↑ G. Hervé, H. Bernard, N. Le Bris, J.-J. Yaouane, H. Handel, L. Toupet: A new route to cyclen, cyclam and homocyclen . In: Tetrahedron Lett. tape 39 , no. 38 , 1998, pp. 6861-6864 , doi : 10.1016 / S0040-4039 (98) 01497-X .
- ^ EK Barefield, A. Bianchi, EJ Billo, PJ Connolly, P. Paoletti, JS Summers, DG van Derveer: Thermodynamic and structural studies of conformational isomers of [Ni (cyclam)] 2+ . In: Inorg. Chem. Band 25 , no. 23 , 1986, pp. 4197-4202 , doi : 10.1021 / ic00243a028 .
- ↑ a b A. Bianchi, M. Micheloni, P. Paoletti: Thermodynamic aspects of the polyazacycloalkane complexes with cations and anions . In: Coord. Chem. Rev. Volume 110 , no. 1 , 1991, p. 17-113 , doi : 10.1016 / 0010-8545 (91) 80023-7 .
- ^ B. Bosnich, ML Tobe, GA Webb: Complexes of nickel (II) with a cyclic tetradentate secondary amine . In: Inorg. Chem. Band 4 , no. 8 , 1965, p. 1109-1112 , doi : 10.1021 / ic50030a004 .
- ↑ P. Tascedda, E. Dunach: Novel electrochemical reactivity of Ni (cyclam) Br 2 : Catalytic carbon dioxide incorporation into epoxides . In: J. Chem. Soc. Chem. Commun. No. 1 , 1995, p. 43-44 , doi : 10.1039 / C39950000043 .
- ^ JD Froelich, CP Kubiak: The homogeneous reduction of CO 2 by [Ni (cyclam)] 2+ : Increased catalytic rates with the addition of a CO scavenger . In: J. Am. Chem. Soc. tape 137 , no. 10 , 2015, p. 3565-3573 , doi : 10.1021 / ja512575v .
- ↑ M. Beley, J.-P. Collin, R. Ruppert, J.-P. Sauvage: Electrocatalytic reduction of CO 2 by Ni Cyclam 2+ in water: Study of the factors affecting the efficiency and the selectivity of the process . In: J. Am. Chem. Soc. tape 108 , no. 24 , 1886, p. 7461-7467 , doi : 10.1021 / ja00284a003 .
- ^ AJ Counsel, AT Jones, MH Todd, PJ Rutledge: A direct method for the N-tetraalkylation of azamacrocycles . In: Beilstein J. Org. Chem. Volume 12 , 2016, p. 2457-2461 , doi : 10.3762 / bjoc.12.239 .
- ↑ H. Stetter, W. Frank: Complex formation with tetraazacycloalkane-N, N ', N ", N" "- tetraacetic acids depending on the ring size . In: Angew. Chem. Band 88 , no. 22 , 1976, p. 760 , doi : 10.1002 / anie.19760882208 .
- ↑ MR Maurya, EJ Zaluzec, SF Pavkovic, AW Herlinger: Alkaline-earth-metal complexes of 1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid, H 4 TETA, and crystal and molecular structure of H 4 TETA 6H 2 O and [Mg (H 2 TETA) (H 2 O) 4 ] · 4H 2 O . In: Inorg. Chem. Band 30 , no. 19 , 1991, p. 3657-3662 , doi : 10.1021 / ic00019a017 .
- ↑ W. Yang, CM Giandomenico, M. Sartori, DA Moore: Facile N-1 protection of cyclam, cyclen and 1,4,7-triazacyclononane . In: Tetrahedron Lett. tape 44 , no. 12 , 2003, p. 2481-2483 , doi : 10.1016 / S0040-4039 (03) 00338-1 .