Creosol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
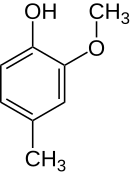
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Creosol | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 8 H 10 O 2 | ||||||||||||||||||
| Brief description |
colorless to yellowish liquid, sweet, spicy, with a slight vanilla odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 138.16 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.092 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
5 ° C |
||||||||||||||||||
| boiling point |
221-222 ° C |
||||||||||||||||||
| Vapor pressure |
3.2 Pa (25 ° C) |
||||||||||||||||||
| pK s value |
10.28 |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.537 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Creosol ( not to be confused with cresol ) is an aromatic compound . It can be seen as a methyl derivative of guaiacol or as a methoxy derivative of p -cresol. It is an isomer to isocreosol , from which it differs only in the position of the methoxy group.
Occurrence
Creosol found mainly in Holzteerkreosot and in Holzteeren , the largest content has Buchholz, but it is also produced in addition to guaiacol in the dry distillation of the guaiacum resin and forms a the guaiacol-like liquid, as well as in pinoresinol . It is also found in vanilla beans , as well as prickly juniper Juniperus oxycedrus and tobacco, as well as many other foods and plants.
presentation
Creosol can be reduced from vanillin with zinc powder and hydrochloric acid . It is also created by the thermal breakdown of ferulic acid . The cleavage of ethers produces 3,4-dihydroxytoluene .
properties
Kreosol is a colorless to yellowish liquid with a sweet, spicy smell that smells slightly of vanilla.
The pK s value of 10.28 is virtually indistinguishable from p -cresol with 10.26; the additional methoxy group has practically no influence.
use
Kreosol is a repellent against tsetse flies . It is used in hair growth preparations and eye lotions, it is an antiseptic and disinfectant. It is also used as a herbicide , in detergents and as a textile abrasive.
Individual evidence
- ^ Entry on FEMA 2671 in the database of the Flavor and Extract Manufacturers Association of the United States .
- ↑ Entry on 2-METHOXY-P-CRESOL in the CosIng database of the EU Commission, accessed on July 10, 2020.
- ↑ entry to cresol in the ChemSpider database of the Royal Society of Chemistry , accessed on 29 July 2016th
- ↑ a b c d e f g data sheet 2-methoxy-4-methylphenol from Sigma-Aldrich , accessed on August 7, 2017 ( PDF ).
- ↑ a b c d e Entry on Creosol in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 29, 2016.
- ↑ George A. Burdock: Encyclopedia of Food and Color Additives . CRC Press, 1997, ISBN 978-0-8493-9412-6 , pp. 1697 ( limited preview in Google Book search).
- ↑ a b George A. Burdock: Fenaroli's Handbook of Flavor Ingredients. Sixth Edition. CRC Press, Boca Raton 2010, ISBN 978-1-420-09086-4 , p. 1198.
- ↑ External identifiers or database links for pinoresinol : CAS number: 487-36-5, PubChem : 73399 , Wikidata : Q3388802 .
- ↑ Emil Abderhalden : Biochemisches Handlexikon. 1st volume, 1st half, Springer-Verlag, 1911, ISBN 978-3-642-51328-2 , p. 645.
- ^ Hermann von Fehling, Carl Hell, Carl Haeussermann, Karl Hugo Bauer (eds.): New Concise Dictionary of Chemistry: based on the Concise Dictionary of Pure and Applied Chemistry published by Liebig, Poggendorf and Wöhler, Kolbe and Fehling . tape 3 . Vieweg, Braunschweig 1878, DNB 560549326 .
- ↑ Daphna Havkin-Frenkel, Faith C. Belanger: Handbook of Vanilla Science and Technology. John Wiley & Sons, 2010, ISBN 978-1-4443-2937-7 .
- ↑ Marina Bährle-Rapp: Springer Lexicon Cosmetics and Body Care. Springer-Verlag, 2007, ISBN 978-3-540-71095-0 , p. 293.
- ↑ Alan Rodgman, Thomas A. Perfetti: The Chemical Components of Tobacco and Tobacco Smoke. CRC Press, 2013, ISBN 978-1-4200-7884-8 , p. 556.
- ^ R. Schwarz, H. Hering: Creosol In: Organic Syntheses . 33, 1953, p. 17, doi : 10.15227 / orgsyn.033.0017 ; Coll. Vol. 4, 1963, p. 203 ( PDF ).
- ↑ Hans-Dieter Belitz, Werner Grosch: Textbook of food chemistry. Springer-Verlag, 1987, ISBN 978-3-662-08308-6 , p. 297.
- ↑ CRC Handbook of Tables for Organic Compound Identification . Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance. 2nd Edition. Springer-Verlag, Wiesbaden 2015, ISBN 978-3-658-07310-7 , p. 67.
- ↑ Ruth Winter: A Consumer's Dictionary of Household, Yard and Office Chemicals. iUniverse, 2007, ISBN 978-0-595-44948-4 , p. 118.
