Copper (II) sulfide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Cu 2+ __ S 2− | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Copper (II) sulfide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | CuS | ||||||||||||||||||
| Brief description |
black solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 95.61 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
4.6 g cm −3 |
||||||||||||||||||
| Melting point |
507 ° C (decomposition) |
||||||||||||||||||
| solubility |
almost insoluble in water (0.33 mg l −1 , 18 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
0.1 mg m −3 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Copper (II) sulphide is a chemical compound of copper and sulfur . It is a black, brittle solid with the ratio formula CuS. Despite this ratio, the compound not only contains Cu 2+ ions, but also consists of a mixture of Cu + and Cu 2+ ions, as well as sulfide ions and disulfide ions . The exact ratio is Cu 2 I Cu II (S 2 ) S.
Occurrence
Copper (II) sulfide occurs naturally as the mineral covellin .
Extraction and presentation
Copper (II) sulfide is produced (in the laboratory) by precipitation from an aqueous solution, for example by introducing hydrogen sulfide .
Highly pure copper (II) sulfide is obtained by reacting a copper (I) sulfide / sulfur mixture at room temperature.
properties
Physical Properties
Copper (II) sulphide is a black, water-insoluble solid that occurs naturally as sulphidic copper ore. It is electrically conductive. The ore copper (II) sulfide is oxidized to copper sulfate in moist air . The compound is stable in dry air at room temperature. If copper (II) sulfide is heated in the absence of air, it decomposes at 507 ° C to copper (I) sulfide and sulfur. The resulting copper sulfide is not built up stoichiometrically and can be better described by the form Cu 2-x S.
Roasting the ore in air, on the other hand, leads to copper (II) oxide and sulfur dioxide.
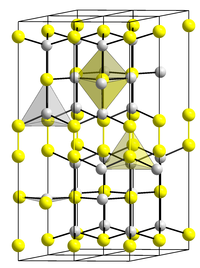
Copper (II) sulfide has a hexagonal crystal structure with the space group P 6 3 / mmc (space group number 194) (a = 3.794 Å, c = 16.33 Å) and an enthalpy of formation of −48.5 kJ / mol.
Chemical properties
Copper (II) sulfide is only soluble in concentrated, oxidizing acids . For example, 3 mol CuS react with 8 mol conc. Nitric acid to copper sulfate (3 mol), nitrogen monoxide (8 mol) and water ( redox reaction ). It is insoluble in dilute acids. In the cation separation process , it is therefore already precipitated in the hydrogen sulfide group at pH 4–5 , dissolved in nitric acid and detected as a copper tetrammine complex with ammonia water ( evidence of cations , detection reaction ).
use
Copper (II) sulfide is used for anti-fouling coatings.
In the 1970s and 1980s, copper (II) sulfide was used as a cathode material in lithium batteries for pacemakers .
Individual evidence
- ↑ a b c d e Entry on copper (II) sulphide in the GESTIS substance database of the IFA , accessed on December 19, 2019 (JavaScript required)
- ↑ a b R. Blachnik, A. Müller: The formation of Cu 2 S from the elements. I. Copper used in the form of powders . In: Thermochimica Acta . tape 361 , no. 1-2 , October 2000, pp. 31-52 , doi : 10.1016 / S0040-6031 (00) 00545-1 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ a b c Georg Brauer (ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 982.



