Land map
| Land map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Country map ( Araschnia levana f. Levana ), spring generation |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Araschnia levana | ||||||||||||
| ( Linnaeus , 1758) |
The land map ( Araschnia levana ) or the land map butterfly is a butterfly (day butterfly ) from the noble butterfly family (Nymphalidae). The species name is derived from Levana , a deity from Roman mythology . The moths of the second generation born in a year are very different from those of the first generation. It was therefore previously assumed that there were two different species. This seasonal dimorphism is controlled by the length of the day during caterpillar development. The pupae from the second generation overwinter. They become butterflies of the first annual generation.
Over the past few decades, the moth has expanded its range in Europe both north and south. The map was voted Butterfly of the Year 2007.
Origin of name
The name of the butterfly is based on the drawing of the undersides of the wings, which are covered with a network of lines and therefore resemble a map . The generic name is derived from the Greek word Arachne for spider and also refers to the web drawing on the underside of the wing. The incorrect spelling with sch instead of ch can no longer be changed due to the nomenclature rules of the ICZN. The name of the spring form levana is derived from the Latin levare (lift, relieve, soften) and refers to the awakening of nature in spring. The name of the summer form prorsa goes back to the Latin word prorsus (forward).
features
Characteristics of the moth
The female moths are larger than the male. The average span for the males is 32 millimeters for the first and 38 millimeters for the second generation; in females, the spans are 38 and 43 millimeters, respectively. The slender body is black-brown, lighter on the underside, with whitish segment rings, and slightly hairy. The moths of both generations have hairy compound eyes and shaggy palps as characteristics of the genus . The antennae , thickened in the shape of a piston, are about half as long as the triangular forewings with their blunt wing tips. The rounded hind wings have a wavy outer edge.
The adults of the spring generation are smaller than those of the summer generation. The moths show a brownish-red to orange-colored base color on the upper side of the wing, which is interspersed with black spots. There are white spots around the tips of the forewings. There is a blue band of spots in the submarginal region . The upper side of the wing thus resembles that of the piebald and mother-of-pearl butterflies .
The upper side of the wings of the summer generation has a black-brown to black, sometimes also blue-black basic color. There is a cream-colored, interrupted band on the hind wings, which continues on the forewings with several spots of the same color. In addition, there are other such spots on the forewings, but they are significantly smaller or line-shaped. The orange color of the spring generation is only present through fine lines on the rear edge of the fore wing and especially on the hind wings in the submarginal and postdiscal region. The dark wing color of the animals with the light band is reminiscent of the small kingfisher ( Limenitis camilla ). With some animals of the summer generation, the levana drawing seems to be very weakly indicated. The band of blue spots in the spring generation is often reduced to a spot in the anal corner in the summer generation.
In contrast to the top of the wing, the underside of the wing of the two generations differs only slightly. The dark basic color, which is lighter in the spring generation, is interrupted by a light line structure of scales on the wing veins . An often darkly speckled light band in the post-disk region of the underside of the wing is more pronounced in the summer generation. On the other hand, in the spring generation, a blurred purple spot with a white core is more pronounced.
In addition to the color and shape of the wings, the physique also varies depending on the generation. In the butterflies of the summer generation, the wing shape is more blunt, and in relation to the body, the wing surface and wing muscles are larger. Due to the stronger flight muscles, the thorax is heavier, as is the abdomen . However, this is easier in relation to the thorax than in the spring generation, whose females lay more eggs that are formed in the abdomen.
For the many deviations from the normal habitus of the two generations that occur in nature, various infrasubspecific names have been assigned, which are of no significance in scientific nomenclature today. Only the occasional intermediate form or third generation, the pattern of which lies between the two other forms, is called A. levana f. called porima . Some authors referred to aberrative butterflies as subprorsa in the case of prorsa if they are darker and have no red pigments on the upper side of the wing, or as superprorsa if they are lighter and have more red pigments than the stem form. Equivalently, darker and lighter individuals in levana were referred to as sublevana and superlevana , respectively. In the case of male aberrative butterflies, which cannot be clearly assigned to one form or the other on the basis of the drawing, an unambiguous assignment through genitalization is possible, as is otherwise used for the unambiguous assignment of species. The sexual organs are examined under a light microscope . The penis tip of the spring generation is long and pointed in contrast to the summer generation, whose penis tip is short and wide.
Sexual dimorphism
In addition to the seasonal dimorphism , the maps also show a weak sexual dimorphism . The females are larger than the respective males in both generations, their abdomen is heavier and their thorax is lighter. The females also have more rounded fore wings. The levana females target have on the hind wings normally two instead of an orange line.
Characteristics of the caterpillars
The black caterpillars have branched thorns on each body segment and are 25 millimeters long and 0.2 grams in weight. The body is finely spotted with white and has interrupted, yellowish-white side and back stripes. The belly legs are yellowish brown. The caterpillars differ from other black butterfly caterpillars, which also live on nettles , such as. B. Peacock butterfly , small fox and admiral , through a pair of thorns on the black head, which is visible from the second larval stage (L2). The shape and size of the head spines differ so greatly in the individual larval stages that they serve as a characteristic for classification. In the second larval stage there are only two thin, unbranched spines; in L3 there are several spines that emerge almost immediately on the head. In L4 the thorns are prickly croissants, which are significantly longer in L5. Occasionally the thorns are colored amber after moulting. These remain colored until pupation or are black again after the next molt.
Seasonal dimorphism
The two forms of the map have long been thought of as two different types due to their different appearance. The spring and summer forms are each immediate descendants of each other. These are two generations that barely overlap in time and that usually do not come into reproductive contact with one another. In the laboratory, however, they can be paired fruitfully.
Story of discovery
Linnaeus described the shapes of the map in 1758 as two types ( Papilio levana and Papilio prorsa ). It was not until 1829 that Christian Friedrich Freyer demonstrated beyond any doubt in breeding experiments that there are two generations of a species that fly at different times of the year. Since this discovery, the phenomenon has been investigated again and again and has also been proven in other insects.
In the first attempts to find the cause of the seasonal dimorphism, the temperature was varied during development. This led to different appearance of individual butterflies, but you never got a majority or even a whole population with the expected appearance. Instead, forms were partially achieved that do not exist in nature. One exception was GW Ruhmer, who carried out cold tests with puppets in 1898. Through cold phases of 0 to 14 days at the beginning of pupal development, he succeeded in breeding a continuous spectrum of forms from prorsa to levana from prorsa pupae . The pupil rest was also extended from 10 to 39 days.
After these attempts did not lead to a clear and reproducible result, the theory of cyclical inheritance was postulated, in which the two forms should alternate again and again. This theory was substantiated when only levana forms were found in eastern Siberia in 1888 . It was assumed that this is the original form and that the prorsa form later developed in the Central European summer climate. Doubts arose when it was established at the beginning of the 20th century that there can be two prorsa generations in a row in a year.
Already at the end of the 19th century there were indications that light could also have an influence on development. In 1954, HJ Müller proved with experiments in which he varied the day length that the day length is the decisive factor for the seasonal dimorphism. The previous temperature tests were not decoupled from the actual factor due to dark refrigerators and the like. Müller managed to breed 14 summer generations in a row in one year. He also succeeded in breeding successive spring generations , but these always required at least three months of cool pupal rest ( diapause ), so that only two generations per year were possible.
The influence of day length
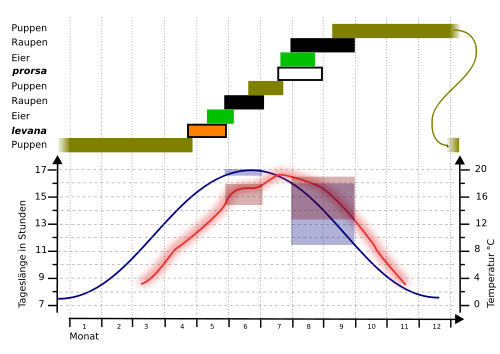
- day length blue
- temperature red
- blue and red rectangles mark the areas in which the caterpillars develop
If the caterpillars are bred under long-day conditions with 18 hours of light and 6 hours of darkness per day, the summer form develops, which hatches after about 14 days. If, on the other hand, the day length is shortened to short day conditions with 14 hours of light and 10 hours of darkness, the spring form develops, which takes a pupil rest and has to hibernate at temperatures below +8 ° C. 12 days under long-day conditions during the caterpillar development are sufficient to maintain the summer form in over 95 percent of the caterpillars. The appearance of the moths therefore depends on the length of the day to which the caterpillars are exposed during their development. The temperature plays only a minor role and the amount of light to which the doll is exposed has no significant influence. Only the temperature at the beginning of the pupal rest, especially in the first 48 hours, still has an influence.
The spring form arises when the caterpillar is exposed to relatively short days in late summer (August, September). The summer form develops when the caterpillar is exposed to long-day conditions in summer (May, June). In terms of day length, the area in which there is a 100 percent change from short-day to long-day butterflies is tightly limited to around one hour. In a population studied by Müller (52 ° north latitude) was the transition point where 50 percent of the caterpillars became A. levana f. prorsa developed at 16 hours. In contrast, it was 17 hours for a population from Freiburg im Breisgau (48 ° north latitude).
The seasonal dimorphism is mainly controlled by hormones from the group of ecdysteroids . The appearance of the moths depends on the time the ecdysteroids are released after pupation. If ecdysteroids are injected into levana dolls, prorsa butterflies hatch. If the dose is reduced accordingly, all intermediate forms can be produced, including those that do not occur in nature. The genes that are responsible for the release of ecdysteroids are activated by the length of the day and temperature. There is no activation in spring due to the short days.
The occasional A. levana f. porima either develop as offspring of the summer form when the day length is already decreasing and fly in the late year or they develop from offspring of the spring form under unfavorable conditions and fly together with the summer generation.
Influence of light on caterpillar survival
In bred caterpillars, these had better survival rates under long-day conditions than under short-day conditions. Over 85 percent of long-day caterpillars made it to pupae, while not even 73 percent of caterpillars bred under short-day conditions made it to pupa. There was also a connection with the melanization of the caterpillars. Darker caterpillars had a slightly better chance of survival, around 5 percent higher, than light-colored caterpillars.
Occurrence
habitat
Land maps prefer moist tall herbaceous meadows with nettles, as can be found in sparse forests, on forest edges and transitional moors . Both the needs of the caterpillars for high humidity and shade and those of the moths for abundant flowering perennials must be met. The moth lives in the flat and hilly areas of the lower elevations and only rarely rises above 1000 meters.
distribution
The distribution area of the map extends from Spain through Central and Northern Europe through Central Asia to Korea and Japan . Its frequency and distribution fluctuates greatly over the decades. Despite a temporary decline in individual regions of Europe, an overall spread and increase within the populated areas has been observed for over 100 years.
In Germany, the map was only represented locally until the 1930s, from the middle of the century it was widespread and often found in places. In the second half of the 20th century it spread north and west in Germany via Lower Saxony and North Rhine-Westphalia to Schleswig-Holstein and the Netherlands to the North Sea coast. The first moths were observed in Jutland ( Denmark ) as early as 1881, but they were not indigenous. In 1955, the northern limit of distribution of indigenous populations was Falster , Lolland and Zealand . It reached Sweden in the 1970s, and it was first seen in Finland in 1973. The map has been established in Finland since 1983 and has continuously expanded its area since then. This area enlargement is not related to global warming , as the expansion also took place to the south. After it appeared in the Pyrenees in 1962 , it spread to Catalonia and was already common near Barcelona in 1993 . In Great Britain the map has not become down-to-earth until now, although it has immigrated several times in the last 100 years.
In particular, the summer generations with a large population are spreading out from the breeding centers and conquering new habitats. In Bohemia , the map disappeared in the first half of the 20th century with the exception of a few remaining populations. After an invasion from other areas began at the end of the 1930s, the map was again frequent here from the 1950s.
Way of life
The caterpillars prefer to eat nettles , but also common burdock chervil . Corresponding to the two generations, the caterpillars are found in May / June and August / September. Due to their physiological differences compared to the spring generation, the adults of the summer generation are generally more mobile. These differences in the generations correspond to the differences that were also found in the forest board game ( Pararge aegeria ) and Argus blue ( Plebejus argus ), when their habitats were connected or fragmented. In the fragmented habitats, mobility was higher and new habitats were more likely to be settled. This goes hand in hand with a slightly lower reproduction rate. Together with the relatively high losses during the diapause due to environmental influences and natural enemies, the first generation is significantly less represented in the year.
Flight time
Two generations fly from April to June and July to August. A partial third generation rarely occurs.
Reproduction
The females feel the leaves with their antennae for a long time before laying their eggs, and later with their abdomen, until they have found a suitable place to lay their eggs. The eggs are then glued to the underside of the fodder plant in the form of individual small columns with up to 10 eggs. On the first day, the entire egg laying consists of an average of 60 eggs, which are divided into three to five columns of the same length and a few shorter columns. If the conditions are favorable, more eggs are deposited on the following days, but with fewer eggs. In the second generation, these are particularly well camouflaged, as the inflorescences of the nettles are already formed at this time of the year and they can easily be mistaken for them at a cursory glance. The eggs, which are initially green, first turn yellow over time, then turn dark until the caterpillars hatch after about 10 days. High humidity is very important at this stage. If this drops below 50 percent, many embryos die. If the conditions are ideal with a warm, humid climate, caterpillars hatch from around 95 percent of the eggs. As with many butterfly caterpillars, the first food for them is the eggshell, which is sometimes only nibbled on or half eaten before the caterpillars turn to the forage plants.
development
The caterpillars initially live socially and spread out more and more as the demand for feed increases. In total, the caterpillars molt four times during their development. The larval stage L3 is the shortest with only about three days and L5 the longest, with about a third of the total time of about 18 days. During the first four larval stages, the two sexes develop equally quickly. Only in the fifth and last instar do the female caterpillars eat longer until pupation. Under short day conditions the caterpillars develop more slowly in all stages than under long day conditions.
The caterpillars that are ready to pupate look for a suitable place to pupate and attach a small pad of spider threads there. They anchor their feeders to it and wait around two days in the prepupal stage. As is usual with tumbling dolls, the skin on the back bursts and the doll becomes visible after turning and bending over. The caterpillar skin usually falls off or remains attached to the end of the body as a small black ball. This process is very dangerous for the caterpillar and crashes lead to losses. If it survives a fall unscathed, it can reattach itself and continue its transformation. Initially, the pupae are green and get darker and darker, with individual, metallic, shiny spots remaining. The summer generation hatches after about 14 to 18 days. The male moths hatch about two to three days before the female ( proterandry ). The subsequent autumn pupae overwinter and then deliver the moths of the spring generation. The moths usually hatch in the morning to be able to dry their wings in the sun. The moths are able to fly after about two to three hours.
Natural enemies
In general, all stages of development of the populations are decimated by predators and parasitoids . Although parasitoids have not yet been observed on the eggs, it can be assumed that polyphagous parasitic wasps also infect the eggs of the map. Caterpillar flies prefer hairy caterpillars, which is why those on the map are only slightly affected. More than 10 percent of the caterpillars are rarely infected. There were Sturmia bella , Compsilura concinnata , Phryxe vulgaris , Phryxe nemea and Bactromyio aurulenta proven.
The older caterpillars often fall prey to birds once they have discovered the clear spots in the eaten nettles. Millipedes , spiders, harvestmen and predatory insects such as predatory bugs and lacewing larvae have been identified as enemies of the caterpillars. Because of the thorns, ants do not seem to be able to prey on the caterpillars. Like many other insects that settle on the flowers to take in nectar, the moths are preyed on by crab spiders when they visit the flowers . But ants and lacewings are also able to prey on sitting map-moths.
Systematics
Within the tribe Nymphalini, which comprises 14 genera, the genus Araschnia forms a monophyletic taxon with the closely related genera Mynes and Symbrenthia .
The genus Araschnia includes seven species, six of which are known to be related to each other. A. davidis has a sister relationship to all other species of the genus. On the next level, A. prorsoides does this with the remaining species. The map is most closely related to A. burejana . These two species have a sister relationship to A. dohertyi , all three in turn form a sister group to A. doris . The relationship of A. oreas is still unclear. A. zhangi Chou , 1994 was synonymous with A. doris in 2010 after genital examinations .
In addition to the map, A. burejana , A. dohertyi , A. doris and A. prorsoides also have a seasonal dimorphism. By A. Davidis no information is available about such, but it could be by Seitz at A. oreas a seasonal form of A. Davidis act.
The following cladogram for the genus is derived from these relationships :
| A. davidis | |||||||||||||||
| A. prorsoides | |||||||||||||||
| A. dohertyi | |||||||||||||||
| A. levana | |||||||||||||||
| A. burejana | |||||||||||||||
| A. doris | |||||||||||||||
Species highlighted in yellow have seasonal dimorphism.
Danger
The map is not threatened and can be very common in suitable habitats. In Germany it is not on the Red List of Endangered Species , only in the federal state of Hamburg is it classified as endangered (3).
Austria sees the map in the national Red List of Threatened Species - albeit in the case of insufficient research - as endangered nationwide (Category 3); the federal state of Styria also reports hazard category 3 for most of the state; the federal state of Tyrol assesses the species as endangered, whereby the state of research is viewed as insufficient; in Tyrol it is endangered and in Vorarlberg extinct or lost, although the status is not clear due to a lack of research.
The map is not endangered in Switzerland.
swell
Individual evidence
- ^ Arnold Spuler: The butterflies of Europe . tape 1 . E. Schweitzerbartsche Verlagbuchhandlung, Stuttgart 1908, p. 20 .
- ↑ a b c d e f g h i j Rolf Reinhardt: Der Landkärtchenfalter. Araschnia levana. Influence of the environment on the change of shape . The New Brehm Library . tape 458 . Ziemsen, 1984, ISSN 0138-1423 .
- ↑ a b c Zdenĕk Fric, Martin Konvička: Generations of the polyphenic butterfly Araschnia levana differ in body design . In: Evolutionary Ecology Research . tape 4 , 2002, p. 1017–1032 ( users.prf.jcu.cz [PDF; 150 kB ; accessed on July 8, 2013]).
- ↑ International Commission on Zoological Nomenclature (Ed.): International Code of Zoological Nomenclature. Fourth Edition . London 1999, ISBN 0-85301-006-4 , Article 29 ( iczn.org [accessed October 12, 2007]). iczn.org ( Memento of the original dated May 24, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ LG Higgins, ND Riley: The butterflies of Europe and Northwest Africa . Paul Parey Publishing House, Hamburg / Berlin 1978, ISBN 3-490-01918-0 .
- ↑ David J. Carter, Brian Hargreaves: Caterpillars and Butterflies of Europe and their Forage Plants . Paul Parey, Hamburg / Berlin 1986, ISBN 3-490-13918-6 , pp. 50 .
- ↑ a b H. J. Müller: The seasonal formation of Araschnia levana, a photoperiodically controlled diapause effect . In: Natural Sciences Berlin . tape 42 . Berlin 1955, p. 134-135 .
- ↑ PB Koch & D. Bückmann: Hormonal control of seasonal morphs by the timing of ecdysteroid release in Araschnia levana (Nymphalidae: Lepidoptera) . In: Journal of Insect Physiology . tape 33 . Elsevier, 1987, ISSN 0022-1910 , pp. 823-829 .
- ↑ a b Jack Windig: Trade-offs between melanization, development time and adult size in Inachis io and Araschnia levane . In: The Genetic Society of Great Britain (Ed.): Heredity . tape 82 , no. 1 . Nature Publishing Group, January 1999, ISSN 0018-067X , p. 57-68 ( nature.com [accessed August 20, 2007]).
- ↑ Manfred Koch : We identify butterflies. Volume 1: Butterfly. 4th enlarged edition. Neumann, Radebeul / Berlin 1966, DNB 457244224 , p. 93.
- ↑ Paul Ralph Ehrlich, Carol L. Boggs, Ward B. Watt: Butterflies - Ecology and Evolution Taking Flight . University of Chicago Press, Chicago 2003, ISBN 0-226-06317-8 .
- ^ Gian-Reto Walther, Peter John Edwards, Conradin A. Burga: "Fingerprints" of Climate Change: Adapted Behavior and Shifting Species Ranges . Springer, 2001, ISBN 0-306-46716-X .
- ↑ Butterfly Species Data - Map - Araschnia levana. In: Butterfly Conservation. Butterfly Conservation Society Great Britain, archived from the original on July 1, 2007 ; Retrieved October 12, 2007 .
- ↑ Otokar Kudrna: Insecta - magazine for entomology and nature protection . Ed .: NABU Federal Technical Committee Entomology. No. 7 . NABU, 2001, ISSN 1431-9721 , p. 31 ( nabu.de [PDF; 3.5 MB ; accessed on October 24, 2007]).
- ^ SY Lang: Taxonomic notes on Araschnia doris Leech, 1892 (Lepidoptera: Nymphalidae) from China. Far East Entomologist, 2010, No. 204, pp. 1-5. biosoil.ru (PDF).
- ↑ Zdenĕk Fric, Martin Konvička & Jan Zrzavy: Red & black or black & white? Phylogeny of the Araschnia butterflies (Lepidoptera: Nymphalidae) and evolution of seasonal polyphenism . In: Journal of Evolutionary Biology . tape 17 . Blackwell Publishing Ltd, 2004, pp. 265-278 , doi : 10.1111 / j.1420-9101.2003.00681.x ( onlinelibrary.wiley.com [PDF; 550 kB ; accessed on February 4, 2009]).
- ↑ The Palaearctic Butterflies . In: Adalbert Seitz (ed.): The large butterflies of the earth . tape 1 . Alfred Kernen, Stuttgart 1909, p. 210 .
- ^ The University of Arizona College of Agriculture and Life Sciences and The University of Arizona Library: Araschnia, Hübner 1819. In: Tree of Life Web Project. Retrieved October 8, 2007 .
- ↑ Butterfly in Hamburg. (PDF) In: Rote List and species directory. Free and Hanseatic City of Hamburg - Authority for Urban Development and the Environment , 2006, accessed on October 12, 2007 .
- ↑ Database query Araschnia levana . In: Red List Austria. Federal Environment Agency Vienna, accessed on February 28, 2010 .
- ↑ Excerpt from the Swiss Red List. In: Faunistic potential of the floodplain object 87 Rüsshalden, Wohlenschwil, AG. Agroscope Reckenholz-Tänikon Research Station, accessed on October 12, 2007 .
literature
- Tom Tolman, Richard Lewington: The butterflies of Europe and Northwest Africa . Franckh-Kosmos Verlags-GmbH & Co, Stuttgart 1998, ISBN 3-440-07573-7 .
- Hans-Josef Weidemann: Butterflies: observe, determine . Naturbuch-Verlag, Augsburg 1995, ISBN 3-89440-115-X .
- Butterflies . Special part: Papilionidae, Pieridae, Nymphalidae. With contribution by René Herrmann and a foreword by Erwin Vetter. In: Günter Ebert, Erwin Rennwald (eds.): The butterflies of Baden-Württemberg . tape 1 . Ulmer Verlag, Stuttgart 1993, ISBN 3-8001-3451-9 .
- Wilhelm Eisenreich, Alfred Handel, Ute E. Zimmer: BLV animal and plant guide for on the go . 17th edition. BLV, Munich, Vienna, Zurich 2000, ISBN 3-405-15608-4 .














