Mesitylene sulfochloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
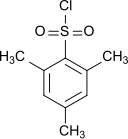
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Mesitylene sulfochloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 11 ClO 2 S | |||||||||||||||
| Brief description |
light gray or cream-colored to white crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 218.72 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point | ||||||||||||||||
| boiling point |
150 ° C at 20 mm Hg |
|||||||||||||||
| solubility |
almost insoluble in water (decomposition), soluble in organic solvents, e.g. B. diethyl ether , toluene , dichloromethane , tetrahydrofuran and in acetonitrile |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Mesitylene sulfochloride is a chemical compound from the group of sulfonic acid chlorides . It is a spatially demanding aromatic sulfonic acid chloride which, because of its reactivity, serves as a starting compound for mesitylenesulfonyl compounds, which are particularly used as biochemical reagents. In technical terms, mesitylene sulfochloride is also abbreviated as MSCl or Mts-Cl.
presentation
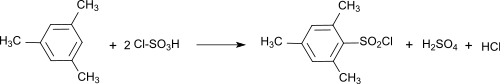
The preparation of mesityl sulfochloride from mesitylene , sulfuryl chloride and aluminum chloride was described as early as 1893.
According to a laboratory procedure, mesitylene sulfochloride is obtained by adding chlorosulfonic acid to mesitylene at temperatures between −15 and 60 ° C and then precipitating the sulfochloride formed by pouring it into ice water and extracting it with dichloromethane . The mesitylene sulfochloride obtained in a crude yield of 80% solidifies to form whitish crystals.
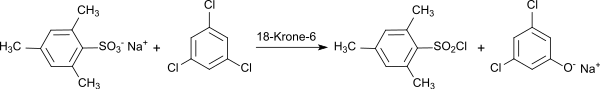
Very high yields (94%) are achieved according to a more recent method in the reaction of the sodium salt of mesitylenesulfonic acid with cyanuric chloride in the presence of the phase transfer catalyst 18-crown-6 in acetone .
properties
Mesitylene sulfochloride is a white to cream-colored hygroscopic solid with an unpleasant, pungent odor that dissolves in water while decomposing. The resulting mesitylenesulfonic acid is highly caustic and corrosive. The substance is readily soluble in a large number of dry organic solvents. For purification, Mts-Cl can be recrystallized from n- hexane or n- pentane .
use
Sulfonamide formation
Mesitylsulfochloride is finding wider application to introduce the mesitylsulfonyl (Mst) protecting group for amino acids and peptides . By reacting with mesitylene sulfochloride, guanidino groups , such as. B. in L-arginine ( protected on the α-amino group with the p-methoxybenzyloxycarbonyl group) with the Mst protective group.
The mesitylenesulfonyl protective group can be split off quantitatively using methanesulfonic acid (MSA), trifluoromethanesulfonic acid (TFMSA) / hydrogen fluoride and with TFMSA / trifluoroacetic acid (TFA) / thioanisole .
In a similar way, the indole group of the amino acid L-tryptophan can be protected with mesitylene sulfochloride to form the corresponding sulfonamide and deprotected with TFMSA / TFA or methanesulfonic acid.
Sulfonate formation
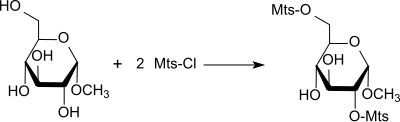
In the presence of pyridine , primary and secondary hydroxyl groups, e.g. B. be blocked in mono- and oligosaccharides by reaction with mesitylene sulfochloride.
With methyl-α-D-glucopyranoside, yields of 98% of the methyl glucoside which is Mst-disubstituted in the 2,6-position are obtained.
Condensation reactions
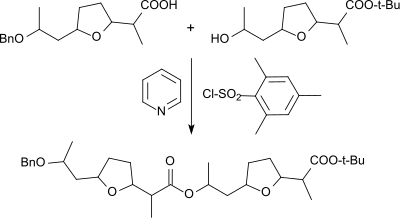
Mesitylene sulfochloride has also been used to activate carboxylic acids in the production of esters , such as. B. the linkage of the selectively protected so-called nonactic acid to the dimer
and then to the tetramer , from which the macrotetrolide antibiotic nonactin is formed by ring closure in the presence of silver ions, which has crown ether- like properties.
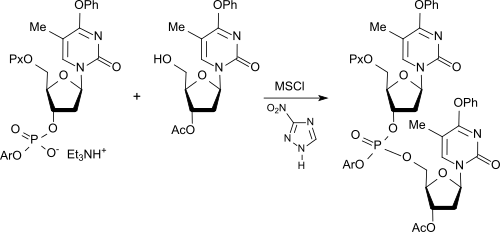
Nucleotide syntheses
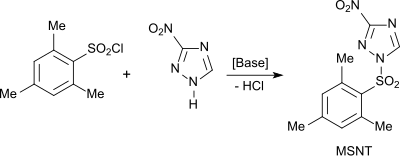
Mesitylene sulfonyl chloride has, despite longer reaction times compared to complex aromatic sulfochlorides, such as. B. 1-mesitylenesulfonyl-3-nitro-triazole (MSNT) or 1- (mesitylsulfonyloxy) -4,6-dinitrobenzotriazole, together with nucleophilic catalysts, such as. B. 3-Nitro-1 H -1,2,4-triazole or 1-hydroxy-4,6-dinitrobenztriazol, advantages in the activation of phosphodiesters for reaction with nucleosides according to the phosphotriester method for the synthesis of oligonucleotides.
Other uses
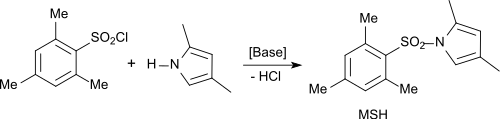
The reaction of mesitylsulfonyl chloride with nitrogen-containing heteroaromatics is suitable for the production of diarylsulfonamides, which act as specific antagonists for the protein EPAC 2 ( exchange protein directly activated by cAMP ), which as a cAMP mediator controls various biological functions.
The sulfonamide with 2,4-dimethylpyrrole was found to be 133 times more effective than cAMP.
The condensing agent 1- (mesityl-2-sulfonyl) -3-nitro-1 H -1,2,4-triazole (MSNT) is obtained from mesitylene sulfonyl chloride and 3-nitro-1 H -1,2,4-triazole , which as an activating agent for nucleotide synthesis is used.
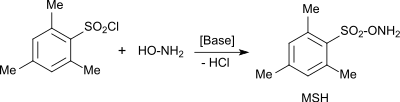
Instead of hydroxylamine-O-sulfonic acid , the more effective (but considerably more expensive) O -esitylenesulfonylhydroxylamine (MSH) is often used for e.g. B. amination reactions are used.
Individual evidence
- ↑ a b c d data sheet mesitylene-2-sulfochloride from Sigma-Aldrich , accessed on September 21, 2015 ( PDF ).
- ↑ a b c d e f data sheet Mesitylene-2-sulfonyl chloride from AlfaAesar, accessed on September 21, 2015 ( PDF )(JavaScript required) .
- ^ A b V. Vaillancourt, MM Cudahy, D. Carbery: Mesitylenesulfonyl Chloride . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2008, doi : 10.1002 / 47084289X.rm049.pub2 .
- ↑ a b C.B. Reese, Z. Pei-Zhuo: Phosphotriester approach to the synthesis of oligonucleotides: a reappraisal . In: J. Chem. Soc., Perkin Trans. 1 . 1993, p. 2291-2301 , doi : 10.1039 / P19930002291 .
- ↑ A. Töhl, O. Eberhard: About the action of sulfuryl chloride on aromatic hydrocarbons . In: Ber. German Chem. Ges. Volume 26 , 1893, pp. 2940-2945 , doi : 10.1002 / cber.189302603118 .
- ^ JR Reid, RF Dufresne, JJ Chapman: Benzenesulfonic acid, 2,4,6-trimethyl-, hydrazide . In: Org. Synth., Coll. Vol. 1998, pp. 281 , doi : 10.15227 / orgsyn.074.0217 .
- ↑ G. Blotny: A new, mild preparation of sulfonyl chlorides . In: Tetrahedron Lett. tape 44 , no. 7 , 2003, p. 1499-1501 , doi : 10.1016 / S0040-4039 (02) 02853-8 .
- ↑ M. Bodanszky, A. Bodanszky: The Practice of Peptide Synthesis, 2nd. Ed. Springer, 1994, doi : 10.1007 / 978-3-642-85055-4 .
- ^ A b H. Yajima, M. Takeyama, J. Kanaki, O. Nishimura, M. Fujino: Studies on Peptides. LXXX. N G -Mesitylene-2-sulfonylarginine . In: Chem. Pharm. Bull. Volume 26 , no. 12 , 1978, p. 3752-3757 , doi : 10.1248 / cpb.26.3752 . , pdf
- ↑ H. Yajima, M. Takeyama, J. Kanaki, K. Mitani: The mesitylene-2-sulphonyl group, an acidolytically removable N G -protecting group for arginine . In: J. Chem. Soc., Chem. Commun. 1978, p. 482-483 , doi : 10.1039 / C39780000482 .
- ↑ N. Fuji, S. Futaki, K. Yasumura, H. Yajima: Studies on Peptides. CXXI. N In -Mesitylenesulfonyl-tryptophan, a New Derivative for Peptide Synthesis . In: Chem. Pharm. Bull. Volume 32 , no. 7 , 1984, pp. 2660-2665 , doi : 10.1248 / cpb.32.2660 . , pdf
- ^ SE Creasey, RD Guthrie: Mesitylenesulphonyl chloride: a selective sulphonating reagent for carbohydrates . In: J. Chem. Soc., Perkin Trans. 1 . 1974, p. 1373-1378 , doi : 10.1039 / P19740001373 .
- ↑ H. Gerlach, K. Oertle, A. Thalmann, S. Servi: Synthesis of Nonactins . In: Helv. Chim. Acta . tape 58 , no. 7 , 1975, p. 2036-2043 , doi : 10.1002 / hlca.19750580718 .
- ↑ CB Reese: The chemical synthesis of oligo- and poly-nucleotides by the phosphotriester approach . In: Tetrahedron . tape 34 , no. 21 , 1978, p. 3143-3179 , doi : 10.1016 / 0040-4020 (78) 87013-6 .
- ↑ H. Chen, T. Tsalkova, OG Chepurny, FC Mei, GG Holz, X. Cheng, J. Zhou: Identification and Characterization of Small Molecules as Potent and Specific EPAC2 Antagonists . In: J. Med. Chem. Volume 56 , no. 3 , 2013, p. 952-962 , doi : 10.1021 / jm3014162 .
- ^ R. Petersen, JF Jensen, TE Nielsen: An Improved Protocol for the Synthesis of 1- (Mesitylenesulfonyl) -3-nitro-1,2,4-triazole (MSNT) . In: Org. Prep. Proc. Int. tape 46 , no. 3 , 2014, p. 267-271 , doi : 10.1080 / 00304948.2014.903145 .
- ↑ J. Mendolia et al .: Preparation, Use, and Safety of O-Mesitylenesulfonylhydroxylamine . In: Org. Process Res. Dev. Band 13 , no. 2 , 2009, p. 263-267 , doi : 10.1021 / op800264p .