Organic nickel compounds
Organic nickel compounds are organometallic compounds that have a nickel - carbon bond. The first compound in this class was nickel tetracarbonyl , a metal carbonyl that Ludwig Mond described as early as 1890 and used in the Mond process to extract nickel. Organic nickel compounds are used as synthesis building blocks in organic chemistry and in chemical vapor deposition . The chemical industry uses organic nickel compounds as catalysts for technical applications such as hydrogenation , carbonylation , hydrocyanation or in the SHOP (Shell Higher Olefin Process).
history
Ludwig Mond , one of the co-founders of Imperial Chemical Industries , investigated the chemistry of nickel tetracarbonyl together with Carl Langer and Friedrich Quincke around 1890 . In their experiments, they treated nickel with carbon monoxide . They received a substance that colored the gas flame of a Bunsen burner greenish-yellow and, when heated in the glass tube, formed a nickel mirror. The compound found could be condensed to a colorless, water-clear liquid with a boiling point of 43 ° C. Ludwig Mond used nickel tetracarbonyl on an industrial scale to extract high-purity nickel using the Mond process. Paul Sabatier later made experiments with nickel, hydrogen and ethene in order to produce the ethene compound analogous to nickel tetracarbonyl. Sabatier discovered hydrogenation by means of nickel contacts, the sought complex trisethene nickel (0) was only synthesized many years later by Günther Wilke .
Walter Reppe discovered a number of homogeneous catalytic reactions in the 1930s , such as the carbonylation of acetylene to acrylic acid using nickel tetracarbonyl as a catalyst. In 1952, Günther Wilke and his colleagues at the Max Planck Institute for Coal Research in Mülheim an der Ruhr discovered the "nickel effect" , the reversal of the conversion of ethene to butene instead of α-olefins , which is influenced by nickel salts, and which is catalyzed by organoaluminum compounds . In addition to the subsequent development of the Ziegler-Natta process , Wilke's group found a number of other reactions of organic nickel compounds, such as the formation of 1,5-cyclooctadiene , vinylcyclohexene or 1,5,9-cyclododecatriene from 1,3-butadiene . Wilke also studied the influence of phosphine ligands on the control of product distribution, which allowed insights into the mechanism of the reactions investigated. In the 1970s, Wilhelm Keim used organic nickel compounds for the oligomerization of ethene to α-olefins in the SHOP process.
links
Organic nickel compounds usually have an oxidation number of 0 or +2. Nickel forms with a number of complexes with ligands such as olefins, carbon monoxide or phosphines. Also carbene or sandwich complexes as Nickelocene are known. Complexes that contain only one type of ligand are called pure or homoleptic complexes. If the complexes contain ligands of different types, the complexes are called mixed or heteroleptic complexes.
Homoleptic nickel complexes
Nickel-olefin complexes
In pure nickel-olefin complexes, nickel has the oxidation state 0. A typical representative of the nickel-olefin complexes is bis [cyclooctadiene (1,5)] nickel (0) . It is produced by reducing nickel salts in the presence of 1,5-cyclooctadiene; other chelating olefin complexes can be obtained in an analogous manner. The complexes can be converted into one another via exchange reactions of the olefin. Bis- [cyclooctadiene- (1,5)] - nickel (0), an 18-electron complex , is suitable for the preparation of many other organic nickel compounds. It reacts with acetylacetone to form cycloocten- (4) -yl- (1) -nickelacetylacetonate .
Nickel-Cyclopentadienyl Complexes
Nickel forms various cyclopentadienyl complexes. A well-known complex is nickelocene , a metallocene that can be prepared from sodium cyclopentadienide and nickel (II) chloride by salt metathesis and has a structure similar to ferrocene . In this complex, nickel has an oxidation number of +2. The complex has a 20-electron structure, with nickel contributing eight electrons and the two cyclopentadienyl anions each contributing six. Therefore the complex is sensitive to oxidizing agents .
Nickelocene reacts with oxidizing agents carbonium ions such as the triphenylmethyl cation [C (C 6 H 5 ) 3 ] + to form a binuclear triple decker complex cation with the composition [Ni 2 (C 5 H 5 ) 3 ] + .
Nickel-allyl complexes
The first homoleptic nickel-allyl complexes were found by Wilke in the reaction of [(trans, trans, trans-1,5,9-cyclododecatriene) Ni (0)] with 1,3-butadiene. The synthesis of bis (π-allyl) nickel (Ni (η 3 -C 3 H 5 ) 2 ) is achieved by reacting [bis (1,5-cyclooctadiene) nickel (0)] with allyl halides such as allyl chloride and further reaction with ammonia .
Heteroleptic Nickel Complexes
Nickel-carbene complexes
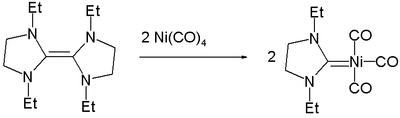
Nickel forms carbene complexes that contain a nickel-carbon double bond .
Reactions
Oligomerizations
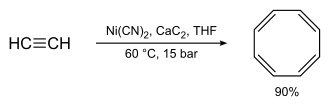
Organic nickel compounds catalyze the oligomerization of alkenes and alkynes . This property was discovered during the development of the Ziegler catalysts as a nickel effect at the Max Planck Institute for Coal Research. Traces of nickel in the autoclave interfered with the build-up reaction in favor of the termination reaction; 1-butene was formed as a product from ethene . Acetylene can be cyclized to cyclooctatetraene using organic nickel complexes .
Formally, this is a [2 + 2 + 2 + 2] cycloaddition. The oligomerization of 1,3-butadiene with ethene to trans-1,4-hexadiene was previously carried out on an industrial scale.
Alkynes can be cyclotrimerized by a formal [2 + 2 + 2] cycloaddition. The method can also be performed using arynes to represent naphthalene used derivatives. The aryne can be generated in situ .
The formation of organic nickel compounds in these reactions is not always obvious, but under suitable experimental conditions these can be represented quantitatively:
The oligomerization of ethene to α-olefins was developed by Wilhelm Keim. The active catalyst is nickel hydride, which is formed by splitting off the cyclooctadiene ligand from the starting olefin complex. By inserting ethene into the nickel-hydrogen bond, oligomers are formed which, through β-elimination, are α-olefins of various chain lengths, but always with an even number of carbon atoms. It is assumed that the diphenylphospinoacetic acid ligand influences the chain length distribution of the resulting olefins.
Coupling reactions
Organic nickel compounds cause coupling reactions between allyl halides and aryl halides . Other coupling reactions using nickel catalysis are the Kumada coupling and the Negishi coupling .
Carbonylation
Organic nickel compounds catalyze the addition of carbon monoxide to alkenes and alkynes. The industrial production of acrylic acid was carried out at times through the reaction of acetylene, carbon monoxide and water at pressures of 40 to 55 bar and temperatures of 160 to 200 ° C.
Hydrocyanation
Organic nickel compounds catalyze the addition of hydrogen cyanide to olefins. If diolefins are used, the synthesis of dinitriles succeeds, which can be further processed into important monomers such as diamides , diamines or diacids. Nickel-phosphine complexes are used as the starting compound, which form nickel-phosphine-allyl complexes in the catalytic cycle.
literature
- Günther Wilke: About organic nickel compounds. In: Pure and Applied Chemistry. 17, 1968, pp. 179-194, doi: 10.1351 / pac196817020179 .
- Günther Wilke: Contributions to organic nickel chemistry. In: Angewandte Chemie. 100, 1988, pp. 189-211, doi: 10.1002 / anie. 19881000113 .
Individual evidence
- ^ Ludwig Mond, Carl Langer, Friedrich Quincke: Action of carbon monoxide on nickel. In: Journal of the Chemical Society, Transactions. 57, 1890, pp. 749-753, doi: 10.1039 / CT8905700749 .
- ^ Günther Wilke: Homogeneous catalysis by transition metal compounds . In: Observation, Experiment and Theory in Science and Medicine . Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 1987, ISBN 3-8047-0912-5 , pp. 275-286.
- ^ Karl Fischer, Klaus Jonas, Günther Wilke: Tris (ethylene) nickel (0). In: Angewandte Chemie. 85, 1973, pp. 620-621, doi: 10.1002 / anged.19730851408 .
- ↑ a b c Wilhelm Keim: Nickel: An element with diverse properties in technically homogeneous catalysis. In: Angewandte Chemie. 102, 1990, pp. 251-260, doi: 10.1002 / anie.19901020305 .
- ↑ Wilhelm Keim: Oligomerization of ethene to α-olefins: Invention and development of the Shell higher olefin process (SHOP). In: Angewandte Chemie. 125, 2013, pp. 12722–12726, doi: 10.1002 / anie.201305308 .
- ↑ a b c Borislav Bogdanović , Michael Kröner, Günther Wilke: Transition Metal Complexes, I. Olefin Complexes of Nickel (0). In: Justus Liebig's Annals of Chemistry. 699, 1966, pp. 1-23, doi: 10.1002 / jlac.19666990102 .
- ↑ Helmut Werner, Albrecht Salzer : The synthesis of a first double sandwich complex: The Dinickeltricyclopentadienyl-Kation. In: Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 2, 1972, pp. 239-248, doi: 10.1080 / 00945717208069606 .
- ↑ G. Wilke, M. Kröner, B. Bogdanovič: An intermediate product in the synthesis of cyclododecatriene from butadiene. In: Angewandte Chemie. 73, 1961, pp. 755-756, doi: 10.1002 / anie.19610732305 .
- ↑ Wolfgang A. Herrmann: N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis N-Heterocyclic Carbenes. In: Angewandte Chemie. 114, p. 1342, doi : 10.1002 / 1521-3757 (20020415) 114: 8 <1342 :: AID-ANGE1342> 3.0.CO; 2-A .
- ↑ Jen-Chieh Hsieh, Chien-Hong Cheng: Nickel-catalyzed cocyclotrimerization of arynes with diynes; a novel method for synthesis of naphthalene derivatives. In: Chemical Communications. 2005, p. 2459, doi: 10.1039 / b415691a .
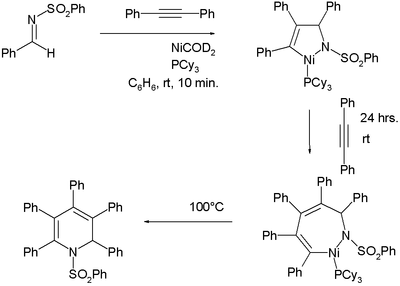
- ↑ Sensuke Ogoshi, Haruo Ikeda, Hideo Kurosawa: Formation of an Aza-nickelacycle by Reaction of an Imine and an Alkyne with Nickel (0): Oxidative Cyclization, Insertion, and Reductive Elimination. In: Angewandte Chemie International Edition. 46, 2007, pp. 4930-4932, doi: 10.1002 / anie.200700688 .
- ↑ B. Taylor: The addition of hydrogen cyanide to α-olefins catalyzed by nickel (0) complexes. In: Journal of Catalysis. 26, 1972, pp. 254-260, doi: 10.1016 / 0021-9517 (72) 90057-7 .