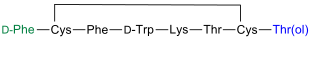Octapeptides
Octapeptides belong to the group of oligopeptides and are made up of eight amino acid building blocks. The term "amino acid building block" comes from the fact that the individual amino acids are linked to one another via peptide bonds . Such a bond is formed by splitting off water between the amino group of one amino acid and the carboxy group of another amino acid.
Like most of the peptides , the octapeptides also occur to a large extent in organisms in which they have important functions. In addition, many octapeptides are pharmacologically active. Many of these compounds are also produced synthetically for this purpose.
Linear octapeptides
If octapeptides have a linear structure, the amino acid building blocks are linked to one another in a chain via seven peptide bonds.
Adrenorphine
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
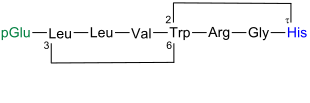
 Adrenorphin comes in the brains of cattle before |
Adrenorphine is also known under the name metorphamide and is one of the opioid peptides . 1983 was first discovered by a Japanese research group led by Kensaku Mizuno (1952 *) from a human pheochromocytoma - tumor of the adrenal medulla receive as well as a group with the Americans Christopher J. Evans (1954 *) from the brain of a cow. It is the first peptide to be found in mammals with an amidated C-terminus. Proenkephalin A is the precursor of this peptide. Adrenorphine binds to μ- and κ-opioid receptors .
Angiotensin II
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 Angiotensin II is in the blood plasma formed |
Angiotensin II (formerly: Hypertensin II) is a peptide hormone that is formed from angiotensin I by the angiotensin-converting enzyme . In 1953 a group led by the American biochemist Leonard T. Skeggs (1918–2002) succeeded in showing that this octapeptide is formed in the blood plasma . Angiotensin II increases blood pressure through vasoconstriction and by increasing the release of aldosterone in the adrenal cortex . In addition, angiotensin II has stimulating effects on glycogenolysis in the liver, on the reuptake of sodium ions in the kidneys, on the release of vasopressin in the brain and on the release of corticotropin in the pituitary gland .
Cyclic octapeptides
The formation of an eighth peptide bond leads to a ring closure , so that the circular shape of the cyclopeptides is created. This is also possible through further covalent bonds, as with hymenistatin-I.
α-amanitin
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 α-Amanitin occurs in the death cap mushrooms , such as z. B. Amanita phalloides in the green amanita mushroom |
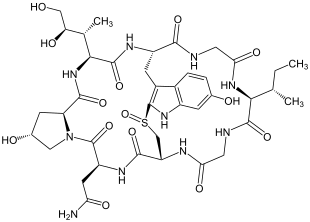
α-Amanitin is one of the amatoxins . These are toxic, bicyclic octapeptides that z. B. occur in the death cap mushrooms . This octapeptide is the first peptide of the amatoxins that was isolated in 1941 by a group led by the German chemist Heinrich Wieland (1877–1957) from the Amanita phalloides green amanita mushroom . It was initially just called Amanitin. It was not until 1949 that another amanitin-like toxin was discovered that it was called α-amanitin.
It is characteristic of the mode of action of α-Amanitin that it is an inhibitor of RNA polymerase II . Because of this property, it should be possible to use this substance in cancer treatment.
Hymenistatin-I
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
Hymenistatin I come in horn silica sponge Hymeniacidon ago |
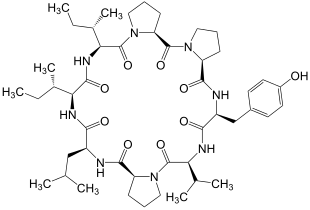
Hymenistatin-I was isolated in 1989 by a team led by the American chemist George R. Pettit (* 1929) from sponges of the genus Hymeniacidon , which belong to the class of horned silica sponges . Similar to ciclosporine , this octapeptide suppresses the humoral and cellular immune response .
Moroidine
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
Natural occurrence |
|
|
 Moroidin occurs in the stinging hairs of the nettle family , such as B. in Dendrocnide moroides |
Moroidine is a bicyclic octapeptide which has an unusual bridge between the N τ atom in the imidazole ring of histidine and the C 2 atom in the indole ring of tryptophan. The second bridge is located between the C 6 atom of the same ring and the C 3 atom in the rest of the leucine.
The molecule is contained in the stinging hairs of plants of the nettle family (e.g. in Laportea moroides and Dendrocnide moroides ), from which it was isolated in 1985 by a working group led by the British biochemist Dudley H. Williams (1937-2010). It appears to be responsible for pain, redness, etc., upon contact with these nettle species. Furthermore, it was found that the Moroidin tubulin - polymerization in derogation and thus as a new class of microtubule - inhibitors is suggested.
Octreotide
| Amino acid sequence according to IUB / IUPAC (with interactive switching field) and structural formula (with green dyed N terminus and blue dyed C terminus) |
|
|
Octreotide is a synthetic somatostatin analog and also known under the name Sandostatin® . It was first synthesized in 1982 by a working group at the pharmaceutical company Sandoz in Switzerland. To the structure of the octapeptide is striking that the amino acid threonine to a nitrogen-containing alcohol , threoninol , reduces was.
The octapeptide is pharmacologically active and, among other things, inhibits the release of the growth hormone somatropin . It is z. B. used in the symptomatic therapy of gastroenteropancreatic tumors such as carcinoids , vipomas and glucagonomas . Octreotide administered subcutaneously has been shown to be effective against cluster headaches . Since octreotide inhibits the internal secretion of gastrointestinal peptide hormones (e.g. cholecystokinin ), side effects such as gallstone formation are possible.
See also
Individual evidence
- ↑ a b c d e f G. D. Fassman (Ed.): Handbook of Biochemistry an Molecular Biology. (= Proteins. Volume I). 3. Edition. CRC Press, Cleveland 1976, ISBN 0-87819-504-1 , pp. 1-108.
- ↑ a b c E. Weber, FS Esch, P. Böhlen, S. Paterson, AD Corbett, AT McKnight, HW Kosterlitz, JD Barchas, CJ Evans: Metorphamide: isolation, structure, and biologic activity of an amidated opioid octapeptide from bovine brain. In: Proceedings of the National Academy of Science of the United States. Volume 80, No. 23, 1983, pp. 7362-7366. doi: 10.1073 / pnas.80.23.7362 .
- ↑ Tohuku University: 'Researchs Information: Kensaku Mizuno.' March 17, 2017. Accessed: July 3, 2017.
- ↑ The information on the year of birth comes from Mr. Evans personally (email contact on July 3, 2017)
- ↑ a b H. Matsuo, A. Miyata, K. Mizuno: Novel C-terminally amidated opioid peptide in human phaeochromocytoma tumor. In: Nature . Volume 35, 1983, pp. 721-723. doi: 10.1038 / 305721a0 .
- ^ A b L. T. Skeggs, WH Marsh, JR Kahn, NP Shumway: The existence of two forms of hypertensin. In: Journal of Experimental Medicine . Volume 99, No. 3, 1954, PMC 2136205 (free full text).
- ↑ E. Braun-Menendez, IH Page: Suggested Revision of Nomenclature - Angiotensin. In: Science . Volume 127, No. 3292, p. 242. doi: 10.1126 / science.127.3292.242-a .
- ^ LT Skeggs, JR Kahn, NP Shumway: The Preparation and Function of the Hypertensin-Converting Enzyme. In: Journal of Experimental Medicine . Volume 103, No. 3, 1956, pp. 295-299. doi: 10.1084 / jem.103.3.295 .
- ^ Case Western Reserve University: In Memoriam: Skeggs worked on artificial kidney In: Campus News. December 2, 2004. Accessed: June 29, 2017.
- ↑ H.-D. Jakubke: peptides. Spektrum Akademischer Verlag , Heidelberg / Berlin / Oxford 1996, ISBN 3-8274-0000-7 , p. 323.
- ↑ a b T. Wieland: Structure and effect of the amatoxins. In: The natural sciences . Vol. 59, No. 6, 1972, pp. 225-231. doi: 10.1007 / BF00610196 .
- ↑ a b H. Wieland, R. Hallermeyer, W. Zilg: About the poisons of the leaf mushroom. VI: Amanitin, the main poison of the death cap mushroom. In: Justus Liebig's Annals of Chemistry . Volume 548, No. 1, 1941, pp. 1-18. doi: 10.1002 / jlac.19415480102 .
- ↑ Th. Wieland, L. Wirth, E. Fischer: About the toxins of the death cap mushroom VII. Β-Amanitin, a third component of the death cap mushroom poison. In: Justus Liebig's Annals of Chemistry . Volume 564, No. 2, 1949, pp. 152-160. doi: 10.1002 / jlac.19495640208
- ↑ H. Faulstich, T. Weiland, L. Fiume: Amatoxins, Phallotoxins, Phallolysin, and Antamanide: The Biologically Active Components of Poisonous Amanita Mushroom. In: CRC Critical Reviews in Biochemistry. Volume 5, No. 3, 1978, pp. 185-260, doi: 10.3109 / 10409237809149870 .
- ^ I. Riede: Switch the Tumor Off: From Genes to Amanita Therapy. In: American Journal of Biomedical Research . Volume 1, No. 4, 2013, pp. 93-107. doi: 10.12691 / ajbr-1-4-5 .
- ^ A b G. R. Pettit, PJ Clewlow, C. Dufresne, DL Doubek, RL Cerny, K. Rützler: Antineoplastic Agents. 193. Isolation and Structure of the Cyclic Peptide Hymenistatin 1. In: Canadian Journal of Chemistry . Volume 68, No. 5, 1990, pp. 708-711. doi: 10.1139 / v90-110 .
- ↑ RS Byars: Waging War on Cancer - Dr. Pettit's Lifelong Quest to Find Cures. Friesenpress, Victoria 2015, ISBN 978-1-4602-5976-4 , p. 1ff.
- ↑ M. Cebrat, Z. Wieczorek, IZ Siemion: Immunosuppressive Activity of Hymenistatin I. In: Peptides . Volume 17, No. 2, 1996, pp. 191-196. doi: 10.1016 / 0196-9781 (95) 02123-X .
- ^ A b c C. Leung, DH Williams, JCJ Barna, S. Foti, PB Oelrichs: Structural Studies on the Peptide Moroidin from Laportea Moroides. In: Tetrahedron . Volume 42, No. 12, 1986, pp. 3333-3348. doi: 10.1016 / S0040-4020 (01) 87397-X .
- ↑ a b c P.B. Oelrichs, DH Williams: Two Naturrally Occurring Toxins Causing Stock Lossed. In: D. Watters, M. Lavin, D. Maguire, J. Pearn (Eds.): Toxins and Targets - Effects of Natural and Synthetic Poisons on Living Cells and Fragile Ecosystems. Harwood Academic Publishers, Chur 1992, ISBN 3-7186-5194-7 , pp. 119-120. ( limited preview in Google Book search)
- ^ DJ Daley: Professor Dudley H. Williams ScD, FRS 1937-2010. In: Rapid Communications in Mass Spectrometry . Volume 25, No. 8, 2011, pp. 117-118. doi: 10.1002 / rcm.4964 .
- ↑ H. Morita, K. Shimbo, H. Shigemori, J. Kobayashi: Antimiotic Activity of Moroidin, a Bicyclic Peptide from the Seed of Celosia argentea. In: Bioorganic & Medicinal Chemistry Letters . Volume 10, No. 5, 2000, pp. 469-471. doi: 10.1016 / S0960-894X (00) 00029-9 .
- ^ W. Bauer, U. Briner, W. Doepfner, R. Haller, R. Huguenin, P. Marbach, TJ Petcher, J. Pless: SMS 201-995: A very potent and selective octapeptide analogue of somatostatin with prolonged action. In: Life Sciences . Volume 31, No. 11, 1982, pp. 1133-1140. doi: 10.1016 / 0024-3205 (82) 90087-X .
- ↑ Entry on threoninol in the ChemSpider database of the Royal Society of Chemistry , accessed on November 18, 2019.
- ^ IUPAC & IUB Joint Commission on Biochemical Nomenclature (eds.): Nomenclature and Symbolism for Amino Acids and Peptides (Recommendations 1983). In: Pure and Applied Chemistry . Volume 56, No. 5, 1984, p. 605. doi: pac198456050595 .
- ↑ Lüllmann, H .; Mohr, K .; Wehling, M .: Pharmacology and Toxicology: Understanding Medicines - Using Medicines Targeted. 15th edition, Georg Thieme Verlag, Stuttgart, 2003, ISBN 3-13-368515-5 , p. 356.
- ↑ S. Herzig, H. Mönig: The drug - Octreotide. In: German Medical Weekly . Volume 120, No. 30, 1995, pp. 1051-1052.
- ↑ a b M.S. Matharu, MJ Levy, K. Meera, J. Goadsby: Subcutaneous Octreotide in Cluster Headache: Randomized Placebo-Controlled Double-Blind Crossover Study. In: Annals of Neurology . Volume 56, No. 4, 2004, pp. 488-494. doi: 10.1002 / ana.20210 .