Synaptic vesicle
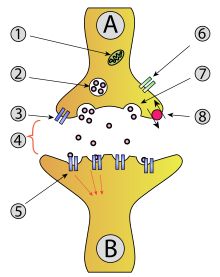
(synaptic transmission)
1 - mitochondrium
2 - synaptic vesicle
3 - presynaptic autoreceptor
4 - synaptic gap with transmitter
5 - postsynaptic receptor
6 - calcium channel
7 - release by exocytosis
8 - active transport , possibly with resumption of neurotransmitters
A synaptic vesicle or synaptic vesicle is a vesicle (vesicle) in the presynaptic terminal of a nerve cell that contains membrane-encased neurotransmitters .
Synaptic vesicles are necessary elements for the transmission of excitation at a chemical synapse between nerve cells and other downstream cells. The synaptic vesicles located in the cytoplasm of the presynaptic region on the cell membrane can fuse with this in response to an action potential and thus release their respective quantum of neurotransmitters into the synaptic gap via exocytosis .
properties
Synaptic vesicles are small, uniformly built organelles of a neuron, whose spherical, membrane-encased fluid space contains a certain amount of specific messenger molecules. Classic low-molecular neurotransmitters are the contents of small synaptic vesicles ( English small synaptic vesicles , SSV). Their mean diameter can vary from synapse to synapse and is between 30 and 50 nm.
In addition, sometimes even more vesicles with denser core region (English are large dense-core vesicles , LDCV) and a diameter of 100-300 nm to be found, mostly as neuromodulators acting neuropeptides contained. It is not uncommon for different types of vesicles with different neurotransmitters or cotransmitters to be found in a presynapse .
If a synaptic vesicle is in close proximity to the cell membrane of the presynaptic region of an axon terminal , after an action potential occurs at the synaptic end button, mediated by intracellular calcium- dependent signals, the vesicle membrane can fuse with the cell membrane. This membrane fusion is a prerequisite for the process called exocytosis, in which the vesicle contents are emptied into the extracellular space. In this way, the contained quantum of neurotransmitters is released from a neuron into the narrow synaptic gap and reaches the postsynaptic region of the downstream cell by diffusion , in whose cell membrane specific receptor proteins for the respective transmitter are built.
The vesicle filling of peptidergic neurons is the product of a biosynthesis in the Golgi apparatus . Simpler transmitters such as acetylcholine can be synthesized in the cytoplasm of the axon terminals and enriched in synaptic vesicles by means of membrane transporters. In addition, a partial resumption of released transmitter or its degradation products is possible in various neurons. By constricting the cell membrane inwards as endocytosis in the previously fused area, it is possible to recycle vesicle membrane ( synaptic vesicle recycling ).
In many neurons, only a part of the synaptic vesicle seems to be mobilized under physiological conditions, the remaining part is called the reserve pool. Under non-physiological conditions - in cell cultures of certain neurons (pyramidal cells from the hippocampal region CA 1 of rats), which individually isolated synapses on themselves ( autapses ) - a considerable part of these reserve vesicles could also break after ten minutes of stimulation with frequencies of 0.2 Hertz Be emptied.
One parameter for the quantity of releasable transmitter is the respective vesicle size. In the human brain , synaptic vesicles of neurons in the primary visual cortex (V1) have a mean diameter of about 40 nm.
Synaptic vesicles consist of over 60 percent of their mass from proteins. Your membrane is interspersed with about 600 transmembrane domains , which take up about a quarter of the membrane area. The membrane of purified synaptic vesicles contains proteins and phospholipids , the phospholipids being composed of approximately 40% phosphatidylcholine , 32% phosphatidylethanolamine , 12% phosphatidylserine , 5% phosphatidylinositol and 10% cholesterol .
The vesicle membrane contains more than 400 different types of proteins, around 40 of which are integral membrane proteins . While the V-ATPase, which builds up an H + gradient, is only available in one or two copies, there are numerous different ion channel proteins, as well as other transport proteins for the uptake of the neurotransmitter molecules into the vesicles (through antiport ). Special proteins enable the processes of exocytosis or endocytosis, such as around 20 different SNARE membrane proteins. For the fusion of the membrane of a synaptic vesicle with the cell membrane, both specific proteins in the vesicle membrane (such as synaptobrevin , synaptophysin and synaptotagmin ) and in the active zone of the presynaptic cell membrane (such as syntaxins and neurexins) are required. Various Rab proteins of their membrane play a role in the intracellular displacements of vesicles .
Membrane transport of neurotransmitters into synaptic vesicles (through antiporters )
| Neurotransmitters | Directed inward | Directed outwards |
|---|---|---|
| Norepinephrine , dopamine , histamine , serotonin and acetylcholine | Neurotransmitter + | 2 H + |
| GABA , glycine | Neurotransmitters | 1 H + |
| Glutamate | Neurotransmitter - + Cl - | 1 H + |
Various toxins inhibit vesicle fusion with the presynaptic cell membrane, e.g. B. batrachotoxin , tetanus toxin , botulinum toxin and alpha-latrotoxin .
history
In 1950 it was observed for the first time that the release of neurotransmitters in frog nerve cells after the presynaptic action potential occurred in larger, discrete units. Under a transmission electron microscope were by George Palade also small vesicles were observed in the presynaptic terminals and colleagues. Based on this, the “vesicle hypothesis” was developed. The term synaptic vesicle was first used in 1954. In 1962, synaptic vesicles called synaptosomes were isolated by cell fractionation . The purification of the synaptosomes was improved by hypoosmotic cell disruption and it was shown that the vesicles contained acetylcholine . About 1000 acetylcholine molecules per vesicle were determined. The release of the vesicle content was replicated in different animal species. The highest concentrations of synaptic vesicles were found in the electric organ of electric rays .
Individual evidence
- ^ A b Y. Park, K. Kim: Short-term plasticity of small synaptic vesicle (SSV) and large dense-core vesicle (LDCV) exocytosis . In: Cellular Signaling . tape 21 , no. October 10 , 2009, ISSN 1873-3913 , p. 1465-1470 , doi : 10.1016 / j.cellsig.2009.02.015 , PMID 19249357 .
- ↑ a b Lei Qu, Yulia Akbergenova, Yunming Hu, Thomas Schikorski: Synapse-to-synapse variation in mean synaptic vesicle size and its relationship with synaptic morphology and function . In: The Journal of Comparative Neurology . tape 514 , no. 4 . Wiley Inter Science, March 2009, ISSN 1096-9861 , pp. 343-352 , doi : 10.1002 / cne.22007 , PMID 19330815 .
- ↑ a b c K. R. Poudel, J. Bai: Synaptic vesicle morphology: a case of protein sorting? In: Current opinion in cell biology . tape February 26 , 2014, ISSN 0955-0674 , p. 28–33 , doi : 10.1016 / j.ceb.2013.09.001 , PMID 24529243 , PMC 4079907 (free full text).
- ^ S. Rizzoli, John M. Bekkers: Synaptic vesicle recycling: steps and principles . In: The EMBO Journal . tape 33 , no. 8 , April 13, 2014, ISSN 0261-4189 , p. 788-822 , doi : 10.1002 / embj.201386357 , PMID 24596248 , PMC 4194108 (free full text).
- ↑ Kaori Ikeda, John M. Bekkers: Counting the number of releasable synaptic vesicles in a presynaptic terminal . In: Proceedings of the National Academy of Sciences . tape 106 , no. 8 , 2009, ISSN 0027-8424 , p. 2945-2950 , doi : 10.1073 / pnas.0811017106 , PMID 19202060 , PMC 2650301 (free full text).
- ↑ F. Benfenati, P. Greengard, J. Brunner, M. Bähler: Electrostatic and hydrophobic interactions of synapsin I and synapsin I fragments with phospholipid bilayers . In: The Journal of Cell Biology . tape 108 , no. 5 , May 1989, ISSN 0021-9525 , pp. 1851-1862 , PMID 2497105 , PMC 2115549 (free full text).
- ↑ S. Takamori, M. Holt, K. Stenius, E. Lemke, M. Grønborg, D. Riedel, H. Urlaub, S. Schenck, B. Brügger, P. Ringler, S. Müller, B. Rammner, F Grater, J. Hub, B. De Groot, G. Mieskes. Y. Moriyama, J. Klingauf, H. Grubmüller, J. Heuser, F. Wieland, R. Jahn: Molecular Anatomy of a Trafficking Organelle. In: Cell. Volume 127, Number 4, November 2006, pp. 831-846, doi: 10.1016 / j.cell.2006.10.030 . PDF (1 MB).
- ↑ Hans-Christian Pape , Armin Kurtz, Stefan Silbernagl (eds.): Physiology . 7th edition. Thieme, Stuttgart 2014, ISBN 978-3-13-796007-2 , p. 146 .
- ^ Paul Fatt, Bernard Katz : Some observations on biological noise . In: Nature . tape 166 , no. 4223 , October 7, 1950, p. 597-598 , doi : 10.1038 / 166597a0 , PMID 14780165 , bibcode : 1950Natur.166..597F .
- ↑ Paul Fatt, Bernard Katz: Spontaneous subthreshold activity at motor nerve endings . In: The Journal of Physiology . tape 117 , no. 1 , May 28, 1952, ISSN 0022-3751 , p. 109-128 , PMID 14946732 , PMC 1392564 (free full text).
- ↑ Sanford L. Palay, George E. Palade: American association of anatomists. Sixty-seventh annual session . In: The Anatomical Record . tape 118 , no. 2 , 1954, Electron microscope study of the cytoplasm of neurons. , S. 275–454 , here p. 336 , doi : 10.1002 / ar.1091180211 ( archive.org ).
- ^ Eduardo DP De Robertis, H. Stanley Bennett: Some Features of the Submicroscopic Morphology of Synapses in Frog and Earthworm . In: The Journal of Biophysical and Biochemical Cytology . tape 1 , no. 1 , January 25, 1955, ISSN 0095-9901 , p. 47-58 , PMID 14381427 , PMC 2223594 (free full text), JSTOR : 1602913 .
- ↑ José del Castillo , Bernard Katz: Quantal components of the end-plate potential . In: The Journal of Physiology . tape 124 , no. 3 , June 28, 1954, ISSN 0022-3751 , p. 560-573 , PMID 13175199 , PMC 1366292 (free full text).
- ↑ José del Castillo, Bernard Katz: Biophysical aspects of neuro-muscular transmission . In: Progress in Biophysics and Biophysical Chemistry . tape 6 , 1956, ISSN 0096-4174 , pp. 121-170 , PMID 13420190 .
- ^ Eduardo DP De Robertis, H. Stanley Bennett: Submicroscopic vesicular component in the synapse. (1954). In: Fed Proc. Volume 13, p. 35.
- ^ EG Gray, VP Whittaker: The isolation of nerve endings from brain . In: Journal of Anatomy . tape 96 , part 1, 1962, ISSN 0021-8782 , p. 79-88.8 , PMID 13901297 , PMC 1244174 (free full text).
- ^ VP Whittaker, IA Michaelson, RJ Kirkland: The separation of synaptic vesicles from disrupted nerve-ending particles . In: Biochemical Pharmacology . tape 12 , no. 3 , March 1963, p. 300-302 , doi : 10.1016 / 0006-2952 (63) 90156-4 .
- ^ VP Whittaker, IA Michaelson, RJ Kirkland: The separation of synaptic vesicles from nerve-ending particles ('synaptosomes') . In: Biochemical Journal . tape 90 , no. 2 , February 1964, p. 293-303 , PMID 5834239 , PMC 1202615 (free full text).
- ^ E. De Robertis, G. Rodriguez de Lores Arnaiz, GL Salganicoff, A. Pellegrino de Iraldi, LM Zieher: Isolation of Synaptic Vesicles and Structural Organization of the Acetylcholine System Within Brain Nerve Endings . In: Journal of Neurochemistry . tape 10 , no. 4 , 1963, ISSN 0022-3042 , pp. 225-235 , doi : 10.1111 / j.1471-4159.1963.tb05038.x .
- ^ VP Whittaker, MN Sheridan: the Morphology and Acetylcholine Content of Isolated Cerebral Cortical Synaptic Vesicles . In: Journal of Neurochemistry . tape 12 , no. 5 , May 1965, ISSN 0022-3042 , pp. 363-372 , doi : 10.1111 / j.1471-4159.1965.tb04237.x , PMID 14333293 .
- ^ WS Wilson, RA Schulz, JR Cooper: The Isolation of Cholinergic Synaptic Vesicles From Bovine Superior Cervical Ganglion and Estimation of Their Acetylcholine Content . In: Journal of Neurochemistry . tape 20 , no. 3 , 1973, ISSN 0022-3042 , pp. 659-667 , doi : 10.1111 / j.1471-4159.1973.tb00026.x , PMID 4574192 .
- ^ DG Jones: The isolation of synaptic vesicles from octopus brain . In: Brain Research . tape 17 , no. 2 , January 20, 1970, ISSN 0006-8993 , pp. 181-193 , doi : 10.1016 / 0006-8993 (70) 90077-6 , PMID 5412681 .
- ↑ M. Israël, J. Gautron, B. Lesbats: Fractionnement de L'organe Electrique de la Torpille. Localization Subcellulaire de L'acetylcholine Subcellular Fractionation of the Electric Organ of Torpedo Marmorata . In: Journal of Neurochemistry . tape 17 , no. October 10 , 1970, ISSN 0022-3042 , pp. 1441-1450 , doi : 10.1111 / j.1471-4159.1970.tb00511.x , PMID 5471906 .
- ^ VP Whittaker, WB Essman, GH Dowe: The isolation of pure cholinergic synaptic vesicles from the electric organs of elasmobranch fish of the family Torpedinidae . In: Biochemical Journal . tape 128 , no. 4 , July 1972, ISSN 0264-6021 , p. 833-845 , PMID 4638794 , PMC 1173903 (free full text).