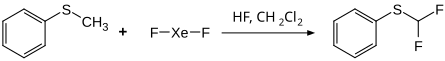Xenon difluoride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| __ Xe 2+ __ F - | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Xenon difluoride | ||||||||||||||||||
| other names |
Xenon (II) fluoride |
||||||||||||||||||
| Ratio formula | XeF 2 | ||||||||||||||||||
| Brief description |
colorless crystals with a penetrating, nauseating odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 169.29 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
4.32 g cm −3 |
||||||||||||||||||
| Melting point |
128.6 ° C |
||||||||||||||||||
| Sublimation point |
114.35 ° C |
||||||||||||||||||
| Vapor pressure |
5.2 hPa (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Fluoride 2.5 mg m −3 inhalable dust content (calculated as fluoride) |
||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
–164 kJ / mol (gas) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Xenon (di) fluoride (XeF 2 ) is a colorless, crystalline noble gas compound . It was first synthesized by Rudolf Hoppe in 1962 as the third compound of a noble gas after xenon hexafluoroplatinate (XePtF 6 ) and xenon tetrafluoride (XeF 4 ) . Under special conditions, xenon reacts with fluorine to form this substance.
presentation
XeF 2 can be produced from the elements with the help of catalysts such as hydrogen fluoride or nickel (II) fluoride under UV radiation:
The reaction is exothermic with a heat of reaction of −164 kJ mol −1 . The compound can also be produced by the reaction of xenon with iodine heptafluoride or by the reaction of xenon with tetrafluoromethane .
Crystals on the order of a few millimeters can be obtained by sublimation.
properties
Physical Properties
At normal pressure and a temperature of 114.35 ° C, it passes directly from the solid to the gaseous state through sublimation . The triple point at which the three phases solid, liquid and gaseous are in equilibrium is at a temperature of 129.03 ° C and a pressure of 1.883 bar. The sublimation pressure function results accordingly ( in Torr, in K) with , and in the temperature range from 273 to 388 K. Here, an evaluation according to Clausius-Clapeyron results in a sublimation enthalpy of 55.2 kJ · mol −1 . The critical temperature is 358 ° C, the critical pressure 93.2 bar, the critical density 1.14 g · cm −3 and the critical volume 149 cm 3 · mol −1 . Xenon difluoride crystals have a tetragonal symmetry in the space group I 4 / mmm (space group no. 139) . The crystal lattice contains isolated XeF 2 molecules; the xenon-fluorine distance is 198 pm. Further polymorphic crystal structures were observed at elevated pressures . At a pressure of 28 GPa there was a conversion into an orthorhombic grid with the space group Immm (No. 71) , at 59 GPa a corresponding grid with the space group Pnma (No. 62) .
Chemical properties
When heated rapidly in air, xenon difluoride breaks down into xenon and fluorine. Despite the negative enthalpy of formation (Δ f H 0 = −163 kJ mol −1 ), this reaction proceeds explosively, as there is a large increase in volume. The compound can react explosively with highly flammable substances such as acetone , dimethyl sulfide , aluminum powder , magnesium powder as well as with grease or paper. The compound reacts with hydrogen at temperatures between 300 ° C and 400 ° C to form xenon and hydrogen fluoride.
Xenon difluoride behaves as a fluoride donor in a fluoride transfer reaction towards strong Lewis acids . With the pentafluorides of arsenic , antimony , bismuth , ruthenium , iridium and platinum , different ionic compounds are formed depending on the mixing ratio.
- with M = As, Sb, Bi, Ru, Ir, Pt
The compound dissolves undissociated in water and has a half-life of 7 hours at 0 ° C.
use
Xenon (II) fluoride is used as a powerful oxidizing and fluorinating agent in organic synthesis. Corresponding fluoroalkanes can be obtained from aliphatic carboxylic acids via a fluorodecarboxylation reaction .
An aromatic C – H bond can be directly substituted by fluorine via electrophilic fluorination. The directing effect of the substitution is determined according to the rules of electrophilic aromatic substitution . For example, anisole is preferably fluorinated in the ortho and para positions, nitrobenzene in the meta position. Methylphenyl sulfide gives a difluorinated product in the reaction with xenon difluor.
Fluorine can be added to alkenes using xenon difluoride . With ethene a mixture of products resulting from 45% 1,2-difluoroethane 35% 1,1-difluoroethane and 1,1,2-trifluoroethane . The conversion is more selective with butadiene . This results in 87% of the 1,2-addition product. The reaction with 2,3-dimethylbutadiene gives only 1,2-difluoro-2,3-dimethyl-3-butene.
The reaction with aromatic ketones and aldehydes in the presence of catalytic amounts of hydrogen fluoride or silicon tetrafluoride leads to a rearrangement to difluoro-substituted ethers. In the presence of boron trifluoride , this rearrangement is not carried out, with fluorination then taking place on the aromatic.
With trifluoroacetic the unstable ester (CF are 3 COO) 2 Xe and (CF 3 COO) XeF formed whose primary decomposition and then to trifluoromethyl radicals hexafluoroethane leads. In the presence of aromatics or heteroaromatics , these can be trifluoromethylated .
A mixture of magnesium and xenon (II) fluoride is interesting as a high-energy material and burns with a 2575 K flame.
Other noble gas compounds
Sometimes other xenon compounds (mainly chlorides, oxides), but also the krypton compound krypton difluoride (KrF 2 ) could be produced. It is also assumed that there must be various radon oxides and halides in addition to xenon . Most noble gas compounds are much more unstable than xenon difluoride and are often highly explosive. The reactivity of the noble gases presumably increases with increasing atomic weight, so that in theory radon difluoride should be somewhat more stable than XeF 2 .
See also
Web links
literature
- Melita Tramsek, Boris Zemva: Synthesis, Properties and Chemistry of Xenon (II) Fluoride. In: Acta Chim. Slov. Volume 53, No. 2, 2006, pp. 105-116. (PDF)
- Xenon (II) fluoride. in e-EROS Encyclopedia of Reagents for Organic Synthesis , 1999-2013, John Wiley and Sons.
Individual evidence
- ↑ a b Xenon difluoride data sheet (PDF) from Merck , accessed on October 8, 2010.
- ↑ a b c d James L. Weeks and Max S. Matheson: Xenon difluoride . In: Henry F. Holtzclaw, Jr. (Ed.): Inorganic Syntheses . tape 8 . McGraw-Hill Book Company, Inc., 1966, pp. 260-264 (English).
- ↑ a b c d David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-98.
- ^ DK Hindermann, WE Falconer: Magnetic Shielding of 19 F in XeF 2 . In: J. Chem. Phys. tape 50 , no. 3 , 1969, p. 1203 , doi : 10.1063 / 1.1671178 , bibcode : 1969JChPh..50.1203H .
- ↑ a b Xenon difluoride data sheet at AlfaAesar, accessed on October 8, 2010 ( PDF )(JavaScript required) .
- ↑ a b c Ralf Steudel : Chemistry of Non-Metals, Syntheses - Structures - Binding - Use. 4th edition. Walter de Gruyter, Berlin / Boston 2014, ISBN 978-3-11-030439-8 , p. 570, (accessed via De Gruyter Online).
- ↑ a b Xenon difluoride data sheet from Sigma-Aldrich , accessed on January 4, 2018 ( PDF ).
- ↑ Erwin Riedel, Christoph Janiak: Inorganic Chemistry . 9th edition. Walter de Gruyter GmbH, Berlin 2015, ISBN 978-3-11-035526-0 , p. 416 .
- ↑ N. Bartlett: Xenon Hexafluoroplatinate (V) Xe + [PtF 6 ] - . In: Proceedings of the Chemical Society. 1962, p. 218; doi: 10.1039 / PS9620000197 .
- ^ Howard H. Claassen, Henry Selig, John G. Malm: Xenon Tetrafluoride. In: Journal of the American Chemical Society. 84, 1962, pp. 3593-3593; doi: 10.1021 / ja00877a042 .
- ^ Rudolf Hoppe , Wolfgang Dähne , H. Mattauch, KM Rödder: Fluorination of Xenon. In: Angewandte Chemie. 74, 1962, p. 903; doi: 10.1002 / anie.19620742213 .
- ^ Egon Wiberg , Nils Wiberg , Arnold F. Holleman : Inorganic Chemistry . 103rd edition. Walter de Gruyter, Berlin / Boston 2017, ISBN 978-3-11-026932-1 , p. 464, (accessed via De Gruyter Online).
- ↑ a b Entry on xenon connections. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2018.
- ↑ a b c d F. Schreiner, GN McDonald, CL Chernick: Vapor pressure and melting points of xenon difluoride and xenon tetrafluoride. In: J. Phys. Chem. 72, 1968, pp. 1162-1166, doi: 10.1021 / j100850a014 .
- ↑ T. Ogrin, B. Zemva, M. Bohinc, J. Slivnik: Critical Constants and Liquid Densities of Xenon Difluoride and Xenon Tetrafluoride. In: J. Chem. Eng. Data 17, 1972, pp. 418-419, doi: 10.1021 / je60055a003 .
- ↑ a b Gang Wu, Xiaoli Huang, Yanping Huang, Lingyun Pan, Fangfei Li, Xin Li, Mingkun Liu, Bingbing Liu, Tian Cui: Confirmation of the Structural Phase Transitions in XeF2 under High Pressure. In: J. Phys. Chem. C 121, 2017, pp. 6264-6271, doi: 10.1021 / acs.jpcc.6b11558 .
- ^ Henry A. Lewy, PA Agron: The Crystal and Molecular Structure of Xenon Difluoride by Neutron Diffraction. In: Journal of the American Chemical Society . 85, 1963, pp. 241-242; doi: 10.1021 / ja00885a037 .
- ↑ a b L. Roth, U. Weller: Hazardous chemical reactions. Entry for xenon difluoride, status 72. Supplementary delivery 3/2014, ecomed Verlag Landsberg / Lech, ISBN 978-3-609-19587-2 .
- ↑ a b c d e f g e-EROS Encyclopedia of Reagents for Organic Synthesis , 1999-2013, John Wiley and Sons, Inc., entry for Xenon (II) fluoride, accessed January 20, 2018 .
- ↑ MB Smith, J. March: March's Advanced Organic Chemistry. 5th edition. John Wiley Sons, New York 2001, p. 707.
- ↑ N.-C. Yang, T.-C. Shieh, ED Feit, CL Chernick: Reactions of xenon fluorides with organic compounds. In: J. Org. Chem. 35, 1970, pp. 4020-4024, doi: 10.1021 / jo00837a001 .
- ↑ B. Zajc, M. Zupan: Fluorination with xenon difluoride. 37. Room-temperature rearrangement of aryl-substituted ketones to difluoro-substituted ethers. In: J. Org. Chem. 55, 1990, pp. 1099-1102, doi: 10.1021 / jo00290a054 .
- ↑ M. Tamura, Y. Matsukawa, H. Quan, J. Mizukado, A. Sakiya: Reaction of carbonyl compounds with xenon difluoride in the presence of silicon tetrafluoride. In: J. Fluor. Chem. 125, 2004, pp. 705-709, doi: 10.1016 / j.jfluchem.2003.11.019 .
- ^ Y. Tanabe, N. Matsuo, N. Ohno: Direct perfluoroalkylation including trifluoromethylation of aromatics with perfluoro carboxylic acids mediated by xenon difluoride. In: J. Org. Chem. 53, 1988, pp. 4582-4585, doi: 10.1021 / jo00254a033 .
- ↑ E.-C. Koch, V. Weiser, E. Roth, S. Kelzenberg: Magnesium / Xenon (II) fluoride (MAX) - A New High Energy Density Material. In: 35th International Pyrotechnics Seminar. Fort Collins, USA, July 2008, ISBN 978-0-9755274-4-3 , p. 695, doi: 10.13140 / 2.1.1718.4961 .



![{\ displaystyle \ mathrm {Xe \ + \ F_2 \ \ xrightarrow [UV light] {Cat.} \ XeF_2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d4f58691a6fb313994062c56e1c82ad8fcf37ef2)








![{\ displaystyle \ mathrm {2 \, XeF_ {2} + MF_ {5} \ rightarrow [Xe_ {2} F_ {3}] [MF_ {6}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a0afbdbe3ea10a78481624b6c3bb016e43c1a659)
![{\ displaystyle \ mathrm {XeF_ {2} + MF_ {5} \ rightarrow [XeF] [MF_ {6}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fd447c9bc5420faa44bb2de1bd7d4b3b0561a31d)
![{\ displaystyle \ mathrm {XeF_ {2} +2 \, MF_ {5} \ rightarrow [XeF] [M_ {2} F_ {11}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bf897923192b4636e38b51e57cc41cd1b860394e)