Β-Endorphin: Difference between revisions
No edit summary |
ISBN formatted. Added the cs1 style template to denote Vancouver ("vanc") citation style, because references contain "vauthors" attribute to specify the list of authors. Removed parameters. | Use this tool. Report bugs. | #UCB_Gadget |
||
| (18 intermediate revisions by 11 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description|Peptide hormone in |
{{short description|Peptide hormone in humans}} |
||
{{cs1 config|name-list-style=vanc}} |
|||
{{DISPLAYTITLE:''beta''-Endorphin}} |
|||
{{lowercase title}} |
|||
{{Use dmy dates|date=June 2018}} |
{{Use dmy dates|date=June 2018}} |
||
{{chembox |
{{chembox |
||
| Line 7: | Line 8: | ||
| ImageFile= beta-endorphin.png |
| ImageFile= beta-endorphin.png |
||
| ImageSize= 250px |
| ImageSize= 250px |
||
| ImageFile2= Beta endorphin 3D stick.png |
|||
| ImageSize2= 250px |
|||
| IUPACName = L-Tyrosylglycylglycyl-L-phenylalanyl-L-methionyl-L-threonyl-L-seryl-L-glutaminyl-L-lysyl-L-seryl-L-glutaminyl-L-threonyl-L-prolyl-L-leucyl-L-valyl-L-threonyl-L-leucyl-L-phenylalanyl-L-lysyl-L-asparaginyl-L-alanyl-L-isoleucyl-L-isoleucyl-L-lysyl-L-asparaginyl-L-alanyl-L-tyrosyl-L-lysyl-L-lysylglycyl-L-glutamine |
| IUPACName = L-Tyrosylglycylglycyl-L-phenylalanyl-L-methionyl-L-threonyl-L-seryl-L-glutaminyl-L-lysyl-L-seryl-L-glutaminyl-L-threonyl-L-prolyl-L-leucyl-L-valyl-L-threonyl-L-leucyl-L-phenylalanyl-L-lysyl-L-asparaginyl-L-alanyl-L-isoleucyl-L-isoleucyl-L-lysyl-L-asparaginyl-L-alanyl-L-tyrosyl-L-lysyl-L-lysylglycyl-L-glutamine |
||
| OtherNames= |
| OtherNames= |
||
| Line 39: | Line 42: | ||
}} |
}} |
||
''' |
'''β-Endorphin''' (''beta''-endorphin) is an [[endogenous]] [[opioid]] [[neuropeptide]] and [[peptide hormone]] that is produced in certain [[neuron]]s within the [[central nervous system]] and [[peripheral nervous system]].<ref name="NHM-Beta-endorphin">{{cite book | vauthors = Malenka RC, Nestler EJ, Hyman SE | veditors = Sydor A, Brown RY | title = Molecular Neuropharmacology: A Foundation for Clinical Neuroscience | year = 2009 | publisher = McGraw-Hill Medical | location = New York | isbn = 978-0-07-148127-4 | pages = 184, 190, 192 | edition = 2nd | chapter = Chapter 7: Neuropeptides | quote= Opioid Peptides<br />β-Endorphin (also a pituitary hormone) ...<br />Opioid peptides are encoded by three distinct genes. These precursors include POMC, from which the opioid peptide β-endorphin and several nonopioid peptides are derived, as discussed earlier; proenkephalin, from which met-enkephalin and leu-enkephalin are derived; and prodynorphin, which is the precursor of dynorphin and related peptides. Although they come from different precursors, opioid peptides share significant amino acid sequence identity. Specifically, all of the well-validated endogenous opioids contain the same four N-terminal amino acids (Tyr-Gly-Gly-Phe), followed by either Met or Leu ... Among endogenous opioid peptides, β-endorphin binds preferentially to μ receptors. ... Shared opioid peptide sequences. Although they vary in length from as few as five amino acids (enkephalins) to as many as 31 (β-endorphin), the endogenous opioid peptides shown here contain a shared N-terminal sequence followed by either Met or Leu.}}</ref> It is one of three [[endorphins]] that are produced in humans, the others of which include [[α-endorphin]] and [[γ-endorphin]].<ref name="Endogenous opioid families - 2012 review">{{cite journal | vauthors = Li Y, Lefever MR, Muthu D, Bidlack JM, Bilsky EJ, Polt R | title = Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins | journal = [[Future Medicinal Chemistry]] | volume = 4 | issue = 2 | date = February 2012 | pmid = 22300099 | pmc = 3306179 | doi = 10.4155/fmc.11.195 |at= [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3306179/table/T1/ Table 1: Endogenous opioid peptides] }}</ref> |
||
There are multiple forms of β-endorphins with the full sequence of [[tyrosine|Tyr]]-[[Glycine|Gly]]-Gly-[[Phenylalanine|Phe]]-[[Methionine|Met]]-[[Threonine|Thr]]-[[Serine|Ser]]-[[glutamic acid|Glu]]-[[Lysine|Lys]]-Ser-[[Glutamine|Gln]]-Thr-[[Proline|Pro]]-[[Leucine|Leu]]-[[Valine|Val]]-Thr-Leu-Phe-Lys-[[Asparagine|Asn]]-[[Alanine|Ala]]-[[Isoleucine|Ile]]-Ile-Lys-Asn-Ala-Tyr-Lys-Lys-Gly-Glu (31 amino acids) denoted as β-endorphin(1-31) and variants truncated to the first 26 and 27 amino acids as β-endorphin(1-26) and β-endorphin(1-27).<ref name="NHM-Beta-endorphin" /><ref>{{cite journal | vauthors = Pilozzi A, Carro C, Huang X | title = Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism | journal = International Journal of Molecular Sciences | volume = 22 | issue = 1 | pages = 338 | date = December 2020 | pmid = 33396962 | pmc = 7796446 | doi = 10.3390/ijms22010338 |doi-access=free }}</ref><ref>[http://www.genome.ad.jp/dbget-bin/www_bget?compound+C02210+C11684+C16041+C16042+C16043+C15890+C15891+C01574+C16135+C01516+-s DBGET]</ref> The first 16 amino acids are identical to α-endorphin. β-Endorphin is considered to be a part of the [[endogenous opioid]] and [[endorphin]] classes of neuropeptides;<ref name="NHM-Beta-endorphin" /> all of the established endogenous opioid peptides contain the same N-terminal amino acid sequence, Tyr-Gly-Gly-Phe, followed by either {{nowrap|-Met}} or {{nowrap|-Leu}}.<ref name="NHM-Beta-endorphin" /> |
|||
Function of β-endorphin has been known to be associated with [[hunger (physiology)|hunger]], thrill, [[pain]], maternal care, sexual behavior, and [[ |
Function of β-endorphin has been known to be associated with [[hunger (physiology)|hunger]], thrill, [[pain]], maternal care, sexual behavior, and [[reward cognition]]. In the broadest sense, β-endorphin is primarily utilized in the body to reduce stress and maintain homeostasis. In behavioral research, studies have shown that β-endorphin is released via [[volume transmission]] into the [[ventricular system]] in response to a variety of stimuli, and [[Novelty|novel stimuli]] in particular.<ref name=":1">{{cite journal | vauthors = Veening JG, Barendregt HP | title = The effects of beta-endorphin: state change modification | journal = Fluids and Barriers of the CNS | volume = 12 | pages = 3 | date = January 2015 | pmid = 25879522 | pmc = 4429837 | doi = 10.1186/2045-8118-12-3 |doi-access=free }}</ref> |
||
==Formation and structure== |
==Formation and structure== |
||
| Line 49: | Line 52: | ||
A significant factor that differentiates β-endorphin from other endogenous opioids is its [[binding affinity|high affinity]] for and lasting effect on [[μ-opioid receptors]].<ref name=":2" /> The structure of β-endorphin in part accounts for this through its resistance to [[proteolytic enzyme]]s, as its secondary structure makes it less vulnerable to degradation.<ref name=":2" /> |
A significant factor that differentiates β-endorphin from other endogenous opioids is its [[binding affinity|high affinity]] for and lasting effect on [[μ-opioid receptors]].<ref name=":2" /> The structure of β-endorphin in part accounts for this through its resistance to [[proteolytic enzyme]]s, as its secondary structure makes it less vulnerable to degradation.<ref name=":2" /> |
||
[[File:Beta Endorphin- Gene to Product Formation Diagram.jpg| |
[[File:Beta Endorphin- Gene to Product Formation Diagram.jpg|left|thumb|This diagram depicts the formation of β-endorphin from the [[proopiomelanocortin]] gene in the pituitary gland. Portions of the second and third exon of this gene make up the proopiomelanocortin protein. The cleavage of the C-terminal end of this protein produces β-lipotropin, which is then cleaved again to form β-endorphin. The proopiomelanocortin protein is also a precursor to other neuropeptides and hormones, such as adrenocorticotropic hormone.]] |
||
[[File:Beta endorphin AA label.svg|right|thumb|A skeletal diagram showing the amino acid sequence of [[beta-endorphin]] with each amino acid labeled.]] |
|||
{{clear}} |
|||
==Function and effects== |
==Function and effects== |
||
| Line 55: | Line 60: | ||
=== Opioid agonist === |
=== Opioid agonist === |
||
β-Endorphin is an agonist of the [[opioid receptor]]s; it preferentially binds to the [[μ-opioid receptor]].<ref name="NHM-Beta-endorphin" /> Evidence suggests that it serves as a primary endogenous [[ligand]] for the [[μ-opioid receptor]],<ref name="NHM-Beta-endorphin" /><ref name="IUPHAR μ-opioid receptor">{{cite web|date=15 March 2017|title=Opioid receptors: μ receptor|url=http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319|access-date=26 May 2017|website=IUPHAR/BPS Guide to Pharmacology|publisher=International Union of Basic and Clinical Pharmacology|quote=Principal endogenous agonists (Human)<br />β-endorphin (POMC, P01189), [Met]enkephalin (PENK, P01210), [Leu]enkephalin (PENK, P01210) ...<br />Comments: β-Endorphin is the highest potency endogenous ligand|vauthors=Borsodi A, Caló G, Chavkin C, Christie MJ, Civelli O, Cox BM, Devi LA, Evans C, Henderson G, Höllt V, Kieffer B, Kitchen I, Kreek MJ, Liu-Chen LY, Meunier JC, Portoghese PS, Shippenberg TS, Simon EJ, Toll L, Traynor JR, Ueda H, Wong YH}}</ref> the same receptor to which the chemicals extracted from [[opium]], such as [[morphine]], derive their [[analgesic]] properties. β-Endorphin has the highest binding affinity of any endogenous opioid for the μ-opioid receptor.<ref name="NHM-Beta-endorphin" /><ref name=":2" /><ref name="IUPHAR μ-opioid receptor" /> Opioid receptors are a class of [[G protein–coupled receptor|G-protein coupled receptors]], such that when β-endorphin or another opioid binds, a signaling cascade is induced in the cell.<ref>{{cite journal|vauthors=Livingston KE, Traynor JR|year=2018|title=Allostery at opioid receptors: modulation with small molecule ligands|journal=British Journal of Pharmacology|volume=175|issue=14|pages=2846–2856|doi=10.1111/bph.13823|pmc=6016636|pmid=28419415}}</ref> |
β-Endorphin is an agonist of the [[opioid receptor]]s; it preferentially binds to the [[μ-opioid receptor]].<ref name="NHM-Beta-endorphin" /> Evidence suggests that it serves as a primary endogenous [[ligand]] for the [[μ-opioid receptor]],<ref name="NHM-Beta-endorphin" /><ref name="IUPHAR μ-opioid receptor">{{cite web|date=15 March 2017|title=Opioid receptors: μ receptor|url=http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319|access-date=26 May 2017|website=IUPHAR/BPS Guide to Pharmacology|publisher=International Union of Basic and Clinical Pharmacology|quote=Principal endogenous agonists (Human)<br />β-endorphin (POMC, P01189), [Met]enkephalin (PENK, P01210), [Leu]enkephalin (PENK, P01210) ...<br />Comments: β-Endorphin is the highest potency endogenous ligand|vauthors=Borsodi A, Caló G, Chavkin C, Christie MJ, Civelli O, Cox BM, Devi LA, Evans C, Henderson G, Höllt V, Kieffer B, Kitchen I, Kreek MJ, Liu-Chen LY, Meunier JC, Portoghese PS, Shippenberg TS, Simon EJ, Toll L, Traynor JR, Ueda H, Wong YH}}</ref> the same receptor to which the chemicals extracted from [[opium]], such as [[morphine]], derive their [[analgesic]] properties. β-Endorphin has the highest binding affinity of any endogenous opioid for the μ-opioid receptor.<ref name="NHM-Beta-endorphin" /><ref name=":2" /><ref name="IUPHAR μ-opioid receptor" /> Opioid receptors are a class of [[G protein–coupled receptor|G-protein coupled receptors]], such that when β-endorphin or another opioid binds, a signaling cascade is induced in the cell.<ref>{{cite journal|vauthors=Livingston KE, Traynor JR|year=2018|title=Allostery at opioid receptors: modulation with small molecule ligands|journal=British Journal of Pharmacology|volume=175|issue=14|pages=2846–2856|doi=10.1111/bph.13823|pmc=6016636|pmid=28419415}}</ref> Acetylation of the N-terminus of β-endorphin, however, inactivates the neuropeptide, preventing it from binding to its receptor.<ref name=":2" /> The opioid receptors are distributed throughout the central nervous system and within the peripheral tissue of neural and non-neural origin. They are also located in high concentrations in the [[Periaqueductal gray]], [[Locus coeruleus]], and the [[Rostral ventromedial medulla]].<ref>{{cite journal|vauthors=Al-Hasani R, Bruchas MR|date=December 2011|title=Molecular mechanisms of opioid receptor-dependent signaling and behavior|journal=Anesthesiology|volume=115|issue=6|pages=1363–81|doi=10.1097/ALN.0b013e318238bba6|pmc=3698859|pmid=22020140}}</ref> |
||
[[Voltage-dependent calcium channel]]s (VDCCs) are important membrane proteins that mediate the depolarization of neurons, and play a major role in promoting the release of neurotransmitters. When endorphin molecules bind to opioid receptors, G proteins activate and dissociate into their constituent Gα and Gβγ sub-units. The Gβγ sub-unit binds to the intracellular loop between the two trans-membrane helices of the VDCC. When the sub-unit binds to the voltage-dependent calcium channel, it produces a voltage-dependent block, which inhibits the channel, preventing the flow of calcium ions into the neuron. Embedded in the cell membrane is also the [[G protein-coupled inwardly-rectifying potassium channel]]. When a Gβγ or Gα(GTP) molecule binds to the C-terminus of the potassium channel, it becomes active, and potassium ions are pumped out of the neuron.<ref>{{cite journal|vauthors=Yamada M, Inanobe A, Kurachi Y|date=December 1998|title=G protein regulation of potassium ion channels|url=http://pharmrev.aspetjournals.org/content/50/4/723|journal=Pharmacological Reviews|volume=50|issue=4|pages=723–60|pmid=9860808}}</ref><ref>{{cite journal|vauthors=Reuveny E, Slesinger PA, Inglese J, Morales JM, Iñiguez-Liuhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY|date=July 1994|title=Activation of the Cloned Muscarinic Potassium Channel by G Protein βγ Subunits|journal=Nature|volume=370|issue=6485|pages=143–146|doi=10.1038/370143a0|pmid=8022483|bibcode=1994Natur.370..143R|s2cid=4345632}}</ref> The activation of the potassium channel and subsequent deactivation of the calcium channel causes membrane [[Hyperpolarization (biology)|hyperpolarization]]. This is when there is a change in the membrane's potential, so that it becomes more negative. The reduction in calcium ions causes a reduction neurotransmitter release because calcium is essential for this event to occur.<ref>{{cite journal|vauthors=Kosten TR, George TP|date=July 2002|title=The neurobiology of opioid dependence: implications for treatment|journal=Science & Practice Perspectives|volume=1|issue=1|pages=13–20|doi=10.1151/spp021113|pmc=2851054|pmid=18567959}}</ref> This means that neurotransmitters such as [[glutamate]] and [[substance P]] cannot be released from the presynaptic terminal of the neurons. These neurotransmitters are vital in the transmission of pain, and as β-Endorphin reduces the release of these substances, there is a strong analgesic effect. |
[[Voltage-dependent calcium channel]]s (VDCCs) are important membrane proteins that mediate the depolarization of neurons, and play a major role in promoting the release of neurotransmitters. When endorphin molecules bind to opioid receptors, G proteins activate and dissociate into their constituent Gα and Gβγ sub-units. The Gβγ sub-unit binds to the intracellular loop between the two trans-membrane helices of the VDCC. When the sub-unit binds to the voltage-dependent calcium channel, it produces a voltage-dependent block, which inhibits the channel, preventing the flow of calcium ions into the neuron. Embedded in the cell membrane is also the [[G protein-coupled inwardly-rectifying potassium channel]]. When a Gβγ or Gα(GTP) molecule binds to the C-terminus of the potassium channel, it becomes active, and potassium ions are pumped out of the neuron.<ref>{{cite journal|vauthors=Yamada M, Inanobe A, Kurachi Y|date=December 1998|title=G protein regulation of potassium ion channels|url=http://pharmrev.aspetjournals.org/content/50/4/723|journal=Pharmacological Reviews|volume=50|issue=4|pages=723–60|pmid=9860808}}</ref><ref>{{cite journal|vauthors=Reuveny E, Slesinger PA, Inglese J, Morales JM, Iñiguez-Liuhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY|date=July 1994|title=Activation of the Cloned Muscarinic Potassium Channel by G Protein βγ Subunits|journal=Nature|volume=370|issue=6485|pages=143–146|doi=10.1038/370143a0|pmid=8022483|bibcode=1994Natur.370..143R|s2cid=4345632}}</ref> The activation of the potassium channel and subsequent deactivation of the calcium channel causes membrane [[Hyperpolarization (biology)|hyperpolarization]]. This is when there is a change in the membrane's potential, so that it becomes more negative. The reduction in calcium ions causes a reduction of neurotransmitter release because calcium is essential for this event to occur.<ref>{{cite journal|vauthors=Kosten TR, George TP|date=July 2002|title=The neurobiology of opioid dependence: implications for treatment|journal=Science & Practice Perspectives|volume=1|issue=1|pages=13–20|doi=10.1151/spp021113|pmc=2851054|pmid=18567959}}</ref> This means that neurotransmitters such as [[glutamate]] and [[substance P]] cannot be released from the presynaptic terminal of the neurons. These neurotransmitters are vital in the transmission of pain, and as β-Endorphin reduces the release of these substances, there is a strong analgesic effect. |
||
=== Pain management === |
=== Pain management === |
||
| Line 66: | Line 71: | ||
===Exercise=== |
===Exercise=== |
||
{{main|Neurobiological effects of physical exercise#β-Endorphin}} |
{{main|Neurobiological effects of physical exercise#β-Endorphin}} |
||
β-Endorphin release in response to exercise has been known and studied since at least the 1980s.<ref name="pmid6091217">{{cite journal | vauthors = Harber VJ, Sutton JR | title = Endorphins and exercise | journal = Sports Medicine | volume = 1 | issue = 2 | pages = 154–71 | date = March–April 1984 | pmid = 6091217 | doi = 10.2165/00007256-198401020-00004 | s2cid = 6435497 }}</ref> Studies have demonstrated that serum concentrations of endogenous opioids, in particular β-endorphin and [[β-lipotropin]], increase in response to both acute exercise and training.<ref name="pmid6091217" /> The release of β-endorphin during exercise is associated with a phenomenon colloquially known in popular culture as a ''[[runner's high]]''.<ref>{{cite web|url=http://www.webmd.com/depression/guide/exercise-depression|title=Exercise and Depression| |
β-Endorphin release in response to exercise has been known and studied since at least the 1980s.<ref name="pmid6091217">{{cite journal | vauthors = Harber VJ, Sutton JR | title = Endorphins and exercise | journal = Sports Medicine | volume = 1 | issue = 2 | pages = 154–71 | date = March–April 1984 | pmid = 6091217 | doi = 10.2165/00007256-198401020-00004 | s2cid = 6435497 }}</ref> Studies have demonstrated that serum concentrations of endogenous opioids, in particular β-endorphin and [[β-lipotropin]], increase in response to both acute exercise and training.<ref name="pmid6091217" /> The release of β-endorphin during exercise is associated with a phenomenon colloquially known in popular culture as a ''[[runner's high]]''.<ref>{{cite web|url=http://www.webmd.com/depression/guide/exercise-depression|title=Exercise and Depression| vauthors = Goldberg J |date=19 February 2014|website=WebMD|access-date=14 July 2014}}</ref> |
||
=== Sunlight === |
|||
There is evidence that β-endorphin is released in response to [[Ultraviolet|ultraviolet radiation]], either through sun exposure or artificial tanning.<ref>{{Cite web |date=2014-06-19 |title=Addicted to the Sun |url=https://hms.harvard.edu/news/addicted-sun |access-date=2023-08-29 |website=hms.harvard.edu |language=en}}</ref> This is thought to contribute to [[Tanning dependence|addiction behavior]] among excessive [[Sun tanning|sunbathers]] and users of [[Indoor tanning|artificial tanning]] despite health risks. |
|||
== Mechanism of action == |
== Mechanism of action == |
||
β-Endorphin acts as an agonist that binds to various types of [[G protein–coupled receptor]]s(GPCRs), most notably to the mu, delta, and kappa opioid receptors. The receptors are responsible for supra-spinal analgesia.{{ |
β-Endorphin acts as an agonist that binds to various types of [[G protein–coupled receptor]]s(GPCRs), most notably to the mu, delta, and kappa opioid receptors. The receptors are responsible for supra-spinal analgesia.{{medical citation needed|date=January 2019}} |
||
== History == |
== History == |
||
Latest revision as of 23:22, 6 March 2024
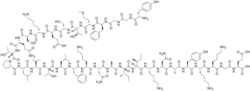
| |

| |
| Names | |
|---|---|
| IUPAC name
L-Tyrosylglycylglycyl-L-phenylalanyl-L-methionyl-L-threonyl-L-seryl-L-glutaminyl-L-lysyl-L-seryl-L-glutaminyl-L-threonyl-L-prolyl-L-leucyl-L-valyl-L-threonyl-L-leucyl-L-phenylalanyl-L-lysyl-L-asparaginyl-L-alanyl-L-isoleucyl-L-isoleucyl-L-lysyl-L-asparaginyl-L-alanyl-L-tyrosyl-L-lysyl-L-lysylglycyl-L-glutamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.056.646 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C158H251N39O46S | |
| Molar mass | 3465.03 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
β-Endorphin (beta-endorphin) is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system.[1] It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.[2]
There are multiple forms of β-endorphins with the full sequence of Tyr-Gly-Gly-Phe-Met-Thr-Ser-Glu-Lys-Ser-Gln-Thr-Pro-Leu-Val-Thr-Leu-Phe-Lys-Asn-Ala-Ile-Ile-Lys-Asn-Ala-Tyr-Lys-Lys-Gly-Glu (31 amino acids) denoted as β-endorphin(1-31) and variants truncated to the first 26 and 27 amino acids as β-endorphin(1-26) and β-endorphin(1-27).[1][3][4] The first 16 amino acids are identical to α-endorphin. β-Endorphin is considered to be a part of the endogenous opioid and endorphin classes of neuropeptides;[1] all of the established endogenous opioid peptides contain the same N-terminal amino acid sequence, Tyr-Gly-Gly-Phe, followed by either -Met or -Leu.[1]
Function of β-endorphin has been known to be associated with hunger, thrill, pain, maternal care, sexual behavior, and reward cognition. In the broadest sense, β-endorphin is primarily utilized in the body to reduce stress and maintain homeostasis. In behavioral research, studies have shown that β-endorphin is released via volume transmission into the ventricular system in response to a variety of stimuli, and novel stimuli in particular.[5]
Formation and structure[edit]
β-Endorphin is found in neurons of the hypothalamus, as well as the pituitary gland. It is derived from β-lipotropin, which is produced in the pituitary gland from a larger peptide precursor, proopiomelanocortin (POMC).[6] POMC is cleaved into two neuropeptides, adrenocorticotropic hormone (ACTH) and β-lipotropin.[7] The formation of β-endorphin is then the result of cleavage of the C-terminal region of β-lipotropin, producing a 31 amino acid-long neuropeptide with an alpha-helical secondary structure. However, POMC also gives rise to other peptide hormones, including α- and γ-melanocyte-stimulating hormone (MSH), resulting from intracellular processing by internal enzymes known as prohormone convertases.
A significant factor that differentiates β-endorphin from other endogenous opioids is its high affinity for and lasting effect on μ-opioid receptors.[6] The structure of β-endorphin in part accounts for this through its resistance to proteolytic enzymes, as its secondary structure makes it less vulnerable to degradation.[6]


Function and effects[edit]
β-Endorphin function is said to be divided into two main categories: local function and global function. Global function of β-endorphin is related to decreasing bodily stress and maintaining homeostasis resulting in pain management, reward effects, and behavioral stability. β-Endorphin in global pathways diffuse to different parts of the body through cerebral spinal fluid in the spinal cord, allowing for β-endorphin release to affect the peripheral nervous system. Localized function of β-endorphin results in release of β-endorphin in different brain regions such as the amygdala or the hypothalamus.[5] The two main methods by which β-endorphin is utilized in the body are peripheral hormonal action[8] and neuroregulation. β-endorphin and other enkephalins are often released with ACTH to modulate hormone system functioning. Neuroregulation by β-endorphin occurs through interference with the function of another neuropeptide, either by direct inhibition of neuropeptide release or induction of a signaling cascade that reduces a neuropeptide's effects.[7]
Opioid agonist[edit]
β-Endorphin is an agonist of the opioid receptors; it preferentially binds to the μ-opioid receptor.[1] Evidence suggests that it serves as a primary endogenous ligand for the μ-opioid receptor,[1][9] the same receptor to which the chemicals extracted from opium, such as morphine, derive their analgesic properties. β-Endorphin has the highest binding affinity of any endogenous opioid for the μ-opioid receptor.[1][6][9] Opioid receptors are a class of G-protein coupled receptors, such that when β-endorphin or another opioid binds, a signaling cascade is induced in the cell.[10] Acetylation of the N-terminus of β-endorphin, however, inactivates the neuropeptide, preventing it from binding to its receptor.[6] The opioid receptors are distributed throughout the central nervous system and within the peripheral tissue of neural and non-neural origin. They are also located in high concentrations in the Periaqueductal gray, Locus coeruleus, and the Rostral ventromedial medulla.[11]
Voltage-dependent calcium channels (VDCCs) are important membrane proteins that mediate the depolarization of neurons, and play a major role in promoting the release of neurotransmitters. When endorphin molecules bind to opioid receptors, G proteins activate and dissociate into their constituent Gα and Gβγ sub-units. The Gβγ sub-unit binds to the intracellular loop between the two trans-membrane helices of the VDCC. When the sub-unit binds to the voltage-dependent calcium channel, it produces a voltage-dependent block, which inhibits the channel, preventing the flow of calcium ions into the neuron. Embedded in the cell membrane is also the G protein-coupled inwardly-rectifying potassium channel. When a Gβγ or Gα(GTP) molecule binds to the C-terminus of the potassium channel, it becomes active, and potassium ions are pumped out of the neuron.[12][13] The activation of the potassium channel and subsequent deactivation of the calcium channel causes membrane hyperpolarization. This is when there is a change in the membrane's potential, so that it becomes more negative. The reduction in calcium ions causes a reduction of neurotransmitter release because calcium is essential for this event to occur.[14] This means that neurotransmitters such as glutamate and substance P cannot be released from the presynaptic terminal of the neurons. These neurotransmitters are vital in the transmission of pain, and as β-Endorphin reduces the release of these substances, there is a strong analgesic effect.
Pain management[edit]
β-Endorphin has been primarily studied for its influence on nociception (i.e., pain perception). β-endorphin modulates pain perception both in the central nervous system and the peripheral nervous system. When pain is perceived, pain receptors (nociceptors) send signals to the dorsal horn of the spinal cord and then up to the hypothalamus through the release of a neuropeptide called substance P.[7][5][15][16] In the peripheral nervous system, this signal causes the recruitment of T-lymphocytes, white blood cells of the immune system, to the area where pain was perceived.[16] T-lymphocytes release β-endorphin in this localized region, allowing it to bind to opioid receptors, causing direct inhibition of substance P.[16][17] In the central nervous system, β-endorphin binds to opioid receptors in the dorsal root and inhibits the release of substance P in the spinal cord, reducing the number of excitatory pain signals sent to the brain.[16][15] The hypothalamus responds to the pain signal by releasing β-endorphin through the periaqueductal grey network, which mainly acts to inhibit the release of GABA, a neurotransmitter which prevents the release of dopamine.[7][15] Thus, the inhibition of GABA release by β-endorphin allows for a greater release of dopamine, in part contributing to the analgesic effect of β-endorphin.[7][15] The combination of these pathways reduces pain sensation, allowing for the body to stop a pain impulse once it has been sent.
β-Endorphin has approximately 18 to 33 times the analgesic potency of morphine,[18] though its hormonal effect is species dependent.[8]
Exercise[edit]
β-Endorphin release in response to exercise has been known and studied since at least the 1980s.[19] Studies have demonstrated that serum concentrations of endogenous opioids, in particular β-endorphin and β-lipotropin, increase in response to both acute exercise and training.[19] The release of β-endorphin during exercise is associated with a phenomenon colloquially known in popular culture as a runner's high.[20]
Sunlight[edit]
There is evidence that β-endorphin is released in response to ultraviolet radiation, either through sun exposure or artificial tanning.[21] This is thought to contribute to addiction behavior among excessive sunbathers and users of artificial tanning despite health risks.
Mechanism of action[edit]
β-Endorphin acts as an agonist that binds to various types of G protein–coupled receptors(GPCRs), most notably to the mu, delta, and kappa opioid receptors. The receptors are responsible for supra-spinal analgesia.[medical citation needed]
History[edit]
β-Endorphin was discovered in camel pituitary extracts by C.H. Li and David Chung.[22] The primary structure of β-endorphin was unknowingly determined 10 years earlier, when Li and colleagues analyzed the sequence of another neuropeptide produced in the pituitary gland, γ-lipotropin. They noticed that the C-terminus region of this neuropeptide was similar to that of some enkephalins, suggesting that it may have a similar function to these neuropeptides. The C-terminal sequence of γ-lipotropin turned out to be the primary sequence of the β-endorphin.[6]
References[edit]
- ^ a b c d e f g Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 7: Neuropeptides". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 184, 190, 192. ISBN 978-0-07-148127-4.
Opioid Peptides
β-Endorphin (also a pituitary hormone) ...
Opioid peptides are encoded by three distinct genes. These precursors include POMC, from which the opioid peptide β-endorphin and several nonopioid peptides are derived, as discussed earlier; proenkephalin, from which met-enkephalin and leu-enkephalin are derived; and prodynorphin, which is the precursor of dynorphin and related peptides. Although they come from different precursors, opioid peptides share significant amino acid sequence identity. Specifically, all of the well-validated endogenous opioids contain the same four N-terminal amino acids (Tyr-Gly-Gly-Phe), followed by either Met or Leu ... Among endogenous opioid peptides, β-endorphin binds preferentially to μ receptors. ... Shared opioid peptide sequences. Although they vary in length from as few as five amino acids (enkephalins) to as many as 31 (β-endorphin), the endogenous opioid peptides shown here contain a shared N-terminal sequence followed by either Met or Leu. - ^ Li Y, Lefever MR, Muthu D, Bidlack JM, Bilsky EJ, Polt R (February 2012). "Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins". Future Medicinal Chemistry. 4 (2). Table 1: Endogenous opioid peptides. doi:10.4155/fmc.11.195. PMC 3306179. PMID 22300099.
- ^ Pilozzi A, Carro C, Huang X (December 2020). "Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism". International Journal of Molecular Sciences. 22 (1): 338. doi:10.3390/ijms22010338. PMC 7796446. PMID 33396962.
- ^ DBGET
- ^ a b c Veening JG, Barendregt HP (January 2015). "The effects of beta-endorphin: state change modification". Fluids and Barriers of the CNS. 12: 3. doi:10.1186/2045-8118-12-3. PMC 4429837. PMID 25879522.
- ^ a b c d e f Smyth DG (May 2016). "60 YEARS OF POMC: Lipotropin and beta-endorphin: a perspective". Journal of Molecular Endocrinology. 56 (4): T13-25. doi:10.1530/JME-16-0033. PMID 26903509.
- ^ a b c d e Dalayeun JF, Norès JM, Bergal S (1993). "Physiology of beta-endorphins. A close-up view and a review of the literature". Biomedicine & Pharmacotherapy. 47 (8): 311–20. doi:10.1016/0753-3322(93)90080-5. PMID 7520295.
- ^ a b Foley KM, Kourides IA, Inturrisi CE, Kaiko RF, Zaroulis CG, Posner JB, Houde RW, Li CH (October 1979). "beta-Endorphin: analgesic and hormonal effects in humans". Proceedings of the National Academy of Sciences of the United States of America. 76 (10): 5377–81. Bibcode:1979PNAS...76.5377F. doi:10.1073/pnas.76.10.5377. PMC 413146. PMID 291954.
- ^ a b Borsodi A, Caló G, Chavkin C, Christie MJ, Civelli O, Cox BM, Devi LA, Evans C, Henderson G, Höllt V, Kieffer B, Kitchen I, Kreek MJ, Liu-Chen LY, Meunier JC, Portoghese PS, Shippenberg TS, Simon EJ, Toll L, Traynor JR, Ueda H, Wong YH (15 March 2017). "Opioid receptors: μ receptor". IUPHAR/BPS Guide to Pharmacology. International Union of Basic and Clinical Pharmacology. Retrieved 26 May 2017.
Principal endogenous agonists (Human)
β-endorphin (POMC, P01189), [Met]enkephalin (PENK, P01210), [Leu]enkephalin (PENK, P01210) ...
Comments: β-Endorphin is the highest potency endogenous ligand - ^ Livingston KE, Traynor JR (2018). "Allostery at opioid receptors: modulation with small molecule ligands". British Journal of Pharmacology. 175 (14): 2846–2856. doi:10.1111/bph.13823. PMC 6016636. PMID 28419415.
- ^ Al-Hasani R, Bruchas MR (December 2011). "Molecular mechanisms of opioid receptor-dependent signaling and behavior". Anesthesiology. 115 (6): 1363–81. doi:10.1097/ALN.0b013e318238bba6. PMC 3698859. PMID 22020140.
- ^ Yamada M, Inanobe A, Kurachi Y (December 1998). "G protein regulation of potassium ion channels". Pharmacological Reviews. 50 (4): 723–60. PMID 9860808.
- ^ Reuveny E, Slesinger PA, Inglese J, Morales JM, Iñiguez-Liuhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY (July 1994). "Activation of the Cloned Muscarinic Potassium Channel by G Protein βγ Subunits". Nature. 370 (6485): 143–146. Bibcode:1994Natur.370..143R. doi:10.1038/370143a0. PMID 8022483. S2CID 4345632.
- ^ Kosten TR, George TP (July 2002). "The neurobiology of opioid dependence: implications for treatment". Science & Practice Perspectives. 1 (1): 13–20. doi:10.1151/spp021113. PMC 2851054. PMID 18567959.
- ^ a b c d Sprouse-Blum AS, Smith G, Sugai D, Parsa FD (March 2010). "Understanding endorphins and their importance in pain management". Hawaii Medical Journal. 69 (3): 70–1. PMC 3104618. PMID 20397507.
- ^ a b c d Luan YH, Wang D, Yu Q, Chai XQ (February 2017). "Action of β-endorphin and nonsteroidal anti-inflammatory drugs, and the possible effects of nonsteroidal anti-inflammatory drugs on β-endorphin". Journal of Clinical Anesthesia. 37: 123–128. doi:10.1016/j.jclinane.2016.12.016. PMID 28235500.
- ^ Plein LM, Rittner HL (2018). "Opioids and the immune system – friend or foe". British Journal of Pharmacology. 175 (14): 2717–2725. doi:10.1111/bph.13750. PMC 6016673. PMID 28213891.
- ^ Loh HH, Tseng LF, Wei E, Li CH (August 1976). "beta-endorphin is a potent analgesic agent". Proceedings of the National Academy of Sciences of the United States of America. 73 (8): 2895–8. Bibcode:1976PNAS...73.2895L. doi:10.1073/pnas.73.8.2895. PMC 430793. PMID 8780.
- ^ a b Harber VJ, Sutton JR (March–April 1984). "Endorphins and exercise". Sports Medicine. 1 (2): 154–71. doi:10.2165/00007256-198401020-00004. PMID 6091217. S2CID 6435497.
- ^ Goldberg J (19 February 2014). "Exercise and Depression". WebMD. Retrieved 14 July 2014.
- ^ "Addicted to the Sun". hms.harvard.edu. 19 June 2014. Retrieved 29 August 2023.
- ^ Li CH, Chung D (April 1976). "Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands". Proceedings of the National Academy of Sciences of the United States of America. 73 (4): 1145–8. Bibcode:1976PNAS...73.1145L. doi:10.1073/pnas.73.4.1145. PMC 430217. PMID 1063395.
External links[edit]
- CID 16132316 from PubChem – β-endorphin
- CID 3081525 from PubChem – β-endorphin (1-9)
- CID 133304 from PubChem – β-endorphin (2-9)
- β-endorphin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
