Alpha radiation
|
α-particle |
|
|---|---|
| properties | |
| electric charge | 2 e (+3.204 · 10 −19 C ) |
| Dimensions | 4.001 506 179 127 (63) u 6.644 657 3357 (20) · 10 −27 kg 7294.299 541 42 (24) m e |
| Resting energy | 3727,379 4066 (11) MeV |
| Spin parity | 0 + |
| Isospin | 0 (z component 0) |
| average lifespan | stable |
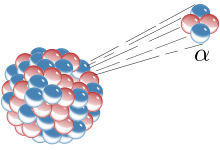
Alpha radiation or α radiation is ionizing radiation that occurs during alpha decay , a type of radioactive decay of atomic nuclei. A radioactive nuclide that emits this radiation is known as an alpha emitter . The name comes from Rutherford 's classification of radiation from radioactive substances into alpha, beta and gamma rays (in the order of increasing penetration power). Alpha radiation is particle radiation because the decaying atomic nucleus ( mother nucleus) sends out a helium- 4 atomic nucleus, which in this case is called an alpha particle , and thus becomes a daughter nucleus .
The symbol for the alpha particle is the small Greek letter α (alpha).
Physics of Alpha Decay
Decay process
The alpha particle is the nucleus of a helium-4 atom, it is a divalent cation of helium. It consists of two protons and two neutrons . The mass number of the nucleus decreases by four units during alpha decay, the atomic number by two units. If X denotes the mother nuclide and Y the daughter nuclide, the energy released during decay, and if mass numbers are written above and ordinal numbers below , as usual , the following applies to alpha decay :
- .
A concrete example is:
- .
The alpha particle leaves the core with an exit speed between about 10,000 km / s and 20,000 km / s, corresponding to a kinetic energy of a few MeV . The initial excess of electrons in the daughter atom that is created is reduced by the recoil of the decay and interaction (charge balance) with the surrounding matter.
Energy spectrum
As with any radioactive decay, alpha decay releases a well-defined amount of energy. It corresponds to the mass that is lost as a mass defect through the process. This energy shows up as the kinetic energy of the alpha particle and the daughter nucleus; In some cases, part of the energy can initially remain as an excited state of the daughter nucleus and then subsequently be dissipated as gamma radiation . The kinetic energy is distributed between the two particles in the inverse proportion of their masses (see kinematics (particle processes) ). The alpha particles emitted by a given nuclide therefore have, unlike, for example, beta decay, only very specific values of the kinetic energy , i.e. that is, its energy spectrum is a line spectrum . This spectrum is characteristic of the respective radionuclide. Its measurement can therefore be used to determine this nuclide.
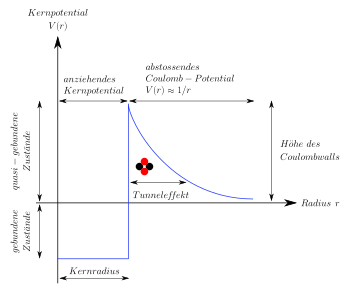
Coulombwall, tunnel effect
On the one hand, the alpha particle is attracted to the nucleus by the strong interaction , but at the same time it is electrically repelled due to the same charges. The stronger nuclear force has a short range, the weaker electrostatic repulsion a long range. Therefore the potential forms a kind of barrier, the Coulomb wall . The wall is higher than the kinetic energy available for the alpha particle. According to classical physics, the alpha particle would therefore be stably bound in the nucleus; however, it can leave it by means of the quantum mechanical tunnel effect . The probability of this per unit of time can be very small. It determines the half-life of the decay. The observed relationship between the half-life and the energy of the emitted alpha particles is described by the Geiger-Nuttall rule .
Radionuclides with alpha decay
Typical alpha emitters occurring in nature are uranium and thorium and their decay products radium and radon . The kinetic energy of an alpha particle is typically on the order of 2 to 5 MeV . Alpha particles from artificially generated nuclides can, however, have energies of over 10 MeV. The alpha energies and half-lives of the individual nuclides can be looked up in the list of isotopes and are given in nuclide maps .
According to the Bethe-Weizsäcker mass formula, the alpha decay results in a positive energy release for all nuclides from mass number 165, because the sum of the masses of the alpha particle and the daughter nucleus calculated in this way is smaller than the mass of the mother nucleus. However, alpha decay has never been observed in many heavy nuclides. However, in the last few decades some nuclides that were previously considered stable have been “exposed” as extremely long-lived alpha emitters, for example 149 Sm , 152 Gd and 174 Hf . It was not until the 2000s that alpha decay with half-lives of a few trillion years was also detected at 180 W and 209 Bi .
proof
In principle, all particle detectors are suitable for detecting alpha radiation, for example for radiation protection purposes . However, the radiation must be able to reach the inside of the detector, the sensitive volume ; a counter tube must have a sufficiently thin film window. Suitable are e.g. B. the usual contamination detection devices . For precise measurements, for example to determine the energy spectrum of the radiation, the radiation source and detector must be in a common vacuum . A semiconductor detector is usually used for this.
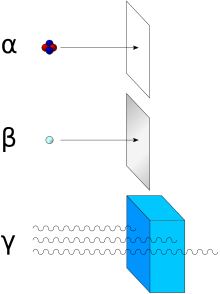
Interaction with matter
Penetration depth, reach
Due to their electrical charge and relatively large mass of 4 u , alpha particles have only a very small penetration depth into matter .
In addition to the respective energy, the range is essentially dependent on the density of the surrounding medium. In air at normal pressure it is approximately 10 cm (at 10 MeV) and is inversely proportional to the air pressure. In the high atmosphere of the earth it is hundreds of kilometers. The cause is the pressure dependence of the free path of the alpha particles, i.e. H. the distance between the collision partners ( molecules ) to which the alpha particles successively transfer their kinetic energy.
The ionization of alpha particles is denser - i.e. H. the number of ions that the particle generates per unit length of its path is much higher - than with beta or gamma radiation, for example . In a cloud chamber , the traces generated by alpha radiation therefore look shorter and thicker compared to those of beta rays of similar energy. The penetration depth of a 5.5 MeV alpha particle in water or organic material is accordingly only about 45 μm. A somewhat thicker sheet of paper or a few centimeters of air are generally sufficient to completely shield alpha radiation.
Biological effect
Alpha radiation that hits the human body from the outside is relatively harmless, as the alpha particles mainly only penetrate into the upper, dead skin layers due to their low penetration depth. On the other hand, an alpha emitter that is incorporated ( incorporated ) in the organism through inhalation or otherwise is very harmful, since its radiation damages living cells. In particular, when an alpha emitter accumulates in an organ, the radiation dose is concentrated in a small space, and u. U. cells from important body cells . The radiation weighting factor for alpha radiation is fixed at 20, while it is 1 for beta and gamma radiation. For the same energy input, 20 times the harmful effect is assumed for alpha radiation.
In radon balneology , a healing effect of low-dose alpha radiation is assumed due to the radon content of some therapeutic baths (e.g. Badgastein ).
Due to the large mass of the alpha particle, the daughter nucleus also receives a noticeable part of the energy released when the alpha decays. This was discovered in 1909 by Lise Meitner and Otto Hahn and corresponds to the kinematics of two-particle decay. The daughter core energies are up to about 200 keV. In the case of incorporated alpha emitters, the recoil cores thus also contribute to tissue damage.
Applications
Isotope battery

Alpha emitters (mainly transuranic elements ) with a relatively short half-life can heat up to red heat due to their own alpha decay. This is possible because almost all the high-energy alpha particles produced during their decay are still held in their interior by their heavy atoms and give them their kinetic energy as heat. If they also generate only a small amount of gamma radiation and their half-life is long enough (usually a few years to decades), the heat given off can be used in radionuclide batteries to generate electrical energy.
smoke detector
Alpha emitters are also used in ionization smoke detectors. You can recognize smoke by measuring the conductivity of the air ionized by alpha rays, as smoke particles reduce the conductivity.
End product helium
If alpha particles have broken down most of their kinetic energy after many collisions in matter, they are so slow that they can trap electrons . This creates the noble gas helium , by far the most common helium isotope, helium-4.
The helium produced from the alpha radiation emitted inside the earth diffuses relatively easily through minerals. In natural gas bubbles it reaches concentrations of a few percent, so that individual natural gas sources can also be used economically for the production of helium.
The helium in the atmosphere continues to rise due to its low density; At altitudes between 700 and 1700 km, helium is the most common gas. A tiny but no longer negligible part of its atoms reaches the escape speed of the earth and escapes the earth's gravitational field forever.
"Alpha rays" from sources other than radioactive
In physics , the term alpha particle is usually used to describe any completely ionized helium-4 nucleus, even if it does not come from a radioactive decay. For example, about 12% of all particles in galactic cosmic rays are alpha particles. This is not surprising as helium is one of the most abundant elements in the universe. However, this part of the cosmic rays never reaches the ground.
Alpha particles can also be artificially created from helium gas in an ion source . If they are in a particle accelerator accelerates the beam is sometimes accordingly Alpha jet called.
Research history
Alpha radiation was the first form of radioactivity to be detected. Antoine Henri Becquerel discovered it in 1896 through the blackening of light-tight packaged photo plates with uranium salts . Further research by Marie Curie and Pierre Curie led, among other things, to the isolation of the uranium decay products radium and polonium and the proof that these are also alpha emitters. The three researchers received the Nobel Prize in Physics in 1903 for these achievements .
In 1898, Ernest Rutherford showed that different types of ionizing radiation could be distinguished by their different penetration capabilities and coined the terms α and β radiation. In 1899, Stefan Meyer , Egon Schweidler and Friedrich Giesel demonstrated the differentiation through different deflections in the magnetic field.
By observing the spectral lines during gas discharge , Rutherford was able to prove the identity of the alpha particles as helium nuclei in 1908.
In 1911, Rutherford used alpha rays for his scattering experiments, which led to the establishment of Rutherford's atomic model .
In 1913, Kasimir Fajans and Frederick Soddy set up the radioactive displacement theorems that determine the nuclide produced during alpha decay .
With alpha rays hitting nitrogen atomic nuclei, Rutherford was able to observe an artificial element transformation for the first time in 1919: oxygen was created in the nuclear reaction 14 N (α, p) 17 O or, more fully written,
- .
In 1928 George Gamow found the quantum mechanical explanation of alpha decay by the tunnel effect , see also Gamow factor .
literature
- Werner Stolz, radioactivity. Basics - Measurement - Applications , Teubner, 5th ed., Wiesbaden 2005, ISBN 3-519-53022-8 .
Nuclear physics
- Theo Mayer-Kuckuk: Kernphysik , Teubner, 6th edition 1994, ISBN 3-519-03223-6
- Klaus Bethge : Nuclear Physics. An introduction ; with 24 tables, 89 exercises with detailed solutions as well as boxes for explanations and a historical overview of the development of nuclear physics. Springer, Berlin 1996, ISBN 3-540-61236-X .
- Jean-Louis Basdevant, James Rich, Michael Spiro: Fundamentals in Nuclear Physics: From Nuclear Structure to Cosmology , Springer, New York 2005, ISBN 0-387-01672-4 .
Research history
- Milorad Mlađenović, The History of Early Nuclear Physics (1896–1931) , World Scientific 1992, ISBN 981-02-0807-3
Radiation protection
- Hanno Krieger: Fundamentals of radiation physics and radiation protection . Teubner, Wiesbaden 2007, ISBN 978-3-8351-0199-9 .
- Claus Grupen: Basic course in radiation protection. Practical knowledge for handling radioactive substances , Springer 2003, ISBN 3-540-00827-6
- James E Martin: Physics for Radiation Protection , John Wiley & Sons, New York 2006, ISBN 0-471-35373-6 .
medicine
- Günter Goretzki: Medical Radiation Science. Physical-technical basics , Urban & Fischer 2004, ISBN 3-437-47200-3
- Thomas Herrmann, Michael Baumann, Wolfgang Dörr: Clinical radiation biology - in a nutshell , Elsevier, Urban and Fischer, Munich 2006, ISBN 3-437-23960-0 .
Web links
- The "Glossary radiation protection" of the Jülich Research Center explained many terms related to ionizing radiation (alpha, beta, gamma radiation, regulations, radiation protection, etc.).
Video
Individual evidence
- ↑ The information about the particle properties of the info box is, unless otherwise stated, taken from the publication of the CODATA Task Group on Fundamental Constants : CODATA Recommended Values. National Institute of Standards and Technology, accessed July 4, 2019 . The numbers in brackets denote the uncertainty in the last digits of the value; this uncertainty is given as the estimated standard deviation of the specified numerical value from the actual value.
- ↑ Cristina Cozzini et al., Detection of the natural? decay of tungsten , Physical Review C (2004), preprint
- ↑ Pierre de Marcillac et al., Experimental detection of alpha particles from the radioactive decay of natural bismuth , Nature 422, 876-878 (April 24, 2003), table of results
- ↑ Harvard Natural Sciences Lecture Demonstrations: α, β, γ Penetration and Shielding. Retrieved February 20, 2020 .
- ^ C. Grupen: Astroparticle Physics , Springer 2005, ISBN 3-540-25312-2 , page 78
- ^ Uranium Radiation and the Electrical Conduction Produced by It . In: Philosophical Magazine . 5th episode, volume 47, number 284, 1899, p. 116 ( doi: 10.1080 / 14786449908621245 ).
- ^ Ernest Rutherford and T. Royds: The Nature of the α Particle from Radioactive Substances. Phil. Mag. 17, 281-6 (1909) online
- ↑ George Gamow (1928): On the quantum theory of the atomic nucleus. Zeitschrift für Physik 51 , p. 204