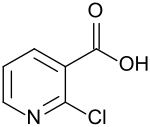
2-chloronicotinic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-chloronicotinic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 4 ClNO 2 | ||||||||||||||||||
| Brief description |
white to beige powdery solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 157.55 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2-chloronicotinic acid is a derivative of pyridine-3-carboxylic acid ( nicotinic acid ). The chlorine atom (–Cl) - substituent in the 2-position - can easily be displaced by nucleophilic aromatic substitution of anionic or neutral nucleophiles . 2-chloronicotinic acid is an intermediate for pharmaceutical and agrochemical active ingredients , including the HIV - drug nevirapine or fungicide boscalid .
Occurrence and representation
The considerable need for 2-chloronicotinic acid has resulted in a large number of synthesis variants which, however, often only contain unsatisfactory yields of pure product, reactants which are unpleasant to handle and complicated by-product streams to be treated.
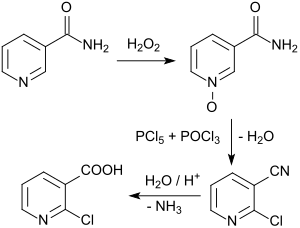
An example is the route via nicotinic acid amide-N-oxide, which is easily accessible from the nicotinic acid amide available in larger quantities by oxidation with 30% hydrogen peroxide in 73 to 82% yield, which is produced with a mixture of phosphorus pentachloride PCl 5 and phosphorus oxychloride POCl 3 in 52 % yield or (more realistic) 35 to 39% yield is converted into the 2-chloronicotinonitrile. The substituted pyridine- N -oxide is reduced to the pyridine derivative in the reaction with phosphorus trichloride (or thionyl chloride ).
This also creates u. a. 6-chloronicotinamide, which is difficult to remove, in considerable quantities. During the acid hydrolysis of the nitrile to the carboxylic acid , the undesired by-product 2-hydroxynicotinic acid is formed. [The gentle amide hydrolysis with amidases that has been developed in recent years completely avoids this by-product.] In addition, larger amounts of phosphate-containing waste water are produced during processing.
The route starting from nicotinic acid is more productive , which is oxidized to the N- oxide with 80% yield and, on halogenation with POCl 3 in the presence of triethylamine, gives 2-CNA in 65% yield after hydrolysis.
The patent literature describes a process variant in which the product 2-CNA still containing 6-chloronicotinic acid is converted by recrystallization from methanol / water 1: 1 into pure 2-chloronicotinic acid in a total yield of 45-50%.
The synthesis starting from 3-cyano-2-pyridone, which is hydrolyzed under alkaline conditions and, after acidification, converted into 2-hydroxynicotinic acid, which is reacted with phosphorus oxychloride to form 2-chloronicotinic acid chloride and which is then hydrolyzed to the end product 2-chloronicotinic acid, is laborious and due to lack of information rather uneconomical to the yields of the process stages.
The disadvantages of the synthesis variants based on pyridine precursors gave rise to the search for alternatives in which intermediate products formed from acyclic precursors are cyclized to 2-chloronicotinic acid derivatives.
One approach is the use of acrolein , ethyl cyanoacetate and ethyl 2-dichlorocyanoacetate, which react with potassium carbonate in 77% yield after 72 hours of reaction time to form an ethyl 2-chloro-2-cyano-5-oxo-pentanoate which reacts with POCl 3 / DMF in 65% yield is regioselectively cyclodehydrated to ethyl 2-chloronicotinate, which still has to be hydrolyzed to give 2-chloronicotinic acid.
Despite inexpensive starting materials, the reaction offers no economic advantages because of unreasonably long reaction times, the accumulation of undesired by-products and an overall yield below 50%.
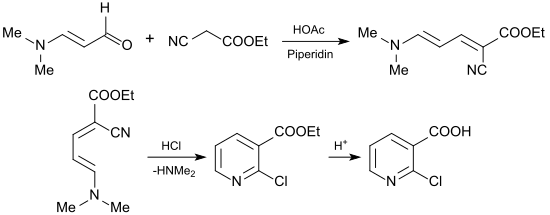
A more suitable starting material is the unstable malondialdehyde in the blocked form of 3-dimethylaminoacrolein (by addition of dimethylamine to propargyl alcohol ) or preferably from the Vilsmeier salt of dimethylformamide , phosgene and vinyl isobutyl ether , which with sodium hydroxide solution gives 3-dimethylaminoacrolein in 86% yield.
In a Knoevenagel reaction , 3- (dimethylamino) acrolein reacts with ethyl cyanoacetate in the presence of glacial acetic acid and piperidine with elimination of water in 91% yield to give 1-cyano-5-dimethylamino-penta-1,3-dienoate (1-cyano-4- dimethylamino-1-ethoxycarbonyl-1,3-butadiene), which by introducing hydrogen chloride in 88% yield to 2-chloronicotinic acid ethyl ester, which can be hydrolyzed practically quantitatively to 2-chloronicotinic acid.
The reaction can also be carried out as a one-pot reaction with slight losses in yield.
The use of the non-polar cyanoacetic acid n-butyl ester instead of the ethyl ester is said to represent an improvement in the process. In the one-pot variant, a yield of 80% is achieved. 2-chloronicotinic acid is obtained practically quantitatively by alkaline hydrolysis of the n- butyl ester.
properties
2-chloronicotinic acid is a white to off-white crystal powder. The substance is only slightly soluble in water, but readily soluble in methanol.
Applications
The chlorine atom in position 2 is easily displaced by nucleophiles. For example, 2-chloronicotinic acid reacts with amines of different constitution in the presence of diisopropylethylamine in water under microwave irradiation in yields of 47–83% to give the corresponding 2-aminonicotinic acid derivatives.
To produce the non-steroidal anti-inflammatory drug nifluminic acid , 2-chloronicotinic acid is reacted with 3-trifluoromethylaniline .
Morniflumate as a prodrug of niflumic acid with better gastrointestinal tolerance is obtained in the reaction of 2-chloronicotinic acid methyl ester (obtained from 2-chloronicotinic acid chloride from 2-CNA) with 3-trifluoromethylaniline in the presence of zinc oxide and iodine and subsequent transesterification with 4- (2-hydroxyethyl) receive.
The nicotinic acid derivative flunixin , which is approved as a non-opioid analgesic in veterinary medicine, is formed when 2-chloronicotinic acid reacts with 2-methyl-3-trifluoromethylaniline.
The herbicide diflufenican is accessible by reacting 2-chloronicotinic acid with 3-trifluoromethylphenol to form 2- (3-trifluoromethylphenoxy) nicotinic acid, reacting it with thionyl chloride to form the acid chloride and reacting it with 2,4-difluoroaniline.
Another herbicide derived from 2-chloronicotinic acid is nicosulfuron , which is prepared in a multi-stage synthesis via the 2-aminosulfonyl-N, N-dimethylnicotinamide.
The antiviral agent nevirapine as the first approved representative of the non-nucleosidic reverse transcriptase inhibitors is based on the conversion of 2-chloronicotinic acid chloride with 2-chloro-3-amino-4-picoline, the nucleophilic substitution of the chlorine atom in the picoline part of the molecule by cyclopropylamine and subsequent ring closure with sodium hydride .
The low overall yield of 25% prompted further process improvements, such as B. the use of 2-chloronicotinonitrile instead of 2-chloronicotinic acid.
The most important derivative of 2-chloronicotinic acid, at least in terms of quantity, is the fungicide boscalid , which is also the largest industrial application of the Suzuki coupling in the reaction of 2-nitrochlorobenzene with 4-chlorophenylboronic acid in the presence of palladium (II) acetate and triphenylphosphine (synthetic route A) represents. In the last stage, the 2- (4'-chlorophenyl) aniline obtained is reacted with the acid chloride of 2-chloronicotinic acid to form the end product.
The decarboxylative aryl coupling with the potassium salt of 2-nitrobenzoic acid and 4-bromo-chlorobenzene (synthesis route B) also provides the important intermediate 2-nitro-4'-chlorobiphenyl.
Individual evidence
- ↑ a b Data sheet 2-chloronicotinic acid from AlfaAesar, accessed on May 25, 2017 ( PDF )(JavaScript required) .
- ↑ a b c d Data sheet 2-chloronicotinic acid for synthesis (PDF) from Merck , accessed on May 25, 2017.
- ↑ a b Entry on 2-Chloronicotinic Acid at TCI Europe, accessed on May 25, 2017.
- ↑ EC Taylor, AJ Crovetti: Nicotinamide-1-oxide In: Organic Syntheses . 37, 1957, p. 63, doi : 10.15227 / orgsyn.037.0063 ; Coll. Vol. 4, 1963, p. 704 ( PDF ).
- ↑ EC Taylor, AJ Crovetti: Pyridine-1-oxides. I. Synthesis of some nicotinic acid derivatives . In: J. Org. Chem. Band 19 , no. 10 , 1954, pp. 1633-1640 , doi : 10.1021 / jo01375a012 .
- ↑ EC Taylor, AJ Crovetti: 2-Chloronicotinonitrile In: Organic Synthesis . 37, 1957, p. 12, doi : 10.15227 / orgsyn.037.0012 ; Coll. Vol. 4, 1963, p. 166 ( PDF ).
- ↑ H. v. Euler, H. Hasselquist, O. Heidenberger: On the knowledge of the N-oxides . In: Chem. Ber. tape 92 , no. 9 , 1959, pp. 2266-2270 , doi : 10.1002 / cber.19590920944 .
- ↑ L.-Q. Jin et al .: Efficient biocatalytic hydrolysis of 2-chloronicotinic amide for production of 2-chloronicotinic acid by recombinant amidase . In: Catal. Commun. tape 38 , 2013, p. 6–9 , doi : 10.1016 / j.catcom.2013.04.004 .
- ↑ E. Kretzschmar: About derivatives of 4-oxo-3,4-dihydropyrido [2,3-d] pyrimidine . In: Pharmacy . tape 35 , no. 5-6 , 1980, pp. 253-256 .
- ↑ Patent US4144238 : Process for the preparation of pure white 2-chloronicotinic acid. Applied on April 4, 1977 , published March 13, 1979 , applicant: Lonza, Ltd., inventor: A. Said.
- ↑ Patent US4081451 : Process for preparing 2-halogeno nicotinic acids. Applied on April 4, 1977 , published March 28, 1978 , applicant: Schering Corp., inventor: J. Mayer.
- ↑ TY Zhang, JR Stout, JG Keay, EFV Scriven, JE Toomey, GL Goe: Regioselective synthesis of 2-chloro-3-pyridinecarboxylates . In: Tetrahedron . tape 51 , no. 48 , 1995, pp. 13177-13184 , doi : 10.1016 / 0040-4020 (95) 00788-A .
- ↑ Patent DE944852 : Process for the preparation of derivatives of 3-amino-acrolein. Registered on June 7, 1956 , published on November 30, 1949 , applicant: Badische Anilin- & Soda-Fabrik AG, inventor: F. Wille.
- ↑ Patent DE19825200C1 : Process for the production of 3-aminoacrolein derivatives. Registered on June 5, 1998 , published on November 18, 1999 , applicant: BASF AG, inventor: D. Grolsch, M. Keil, H. Isak.
- ↑ Patent US4987232 : Preparation of 2-chloropyridine 3-carboxylic acid esters. Applied on December 5, 1989 , published on January 22, 1991 , applicant: Shell Internationale Research Maatschappij, BV, inventor: L. Schröder.
- ↑ Patent WO2000007989A1 : Process for the production of 2-halonicotinic acid derivatives and 2-chloronicotinic acid n-butyl ester as an intermediate. Registered on July 14, 1999 , published on February 17, 2000 , applicant: BASF AG, inventor: D. Grolsch, M. Keil, H. Isak, H. Mayer.
- ↑ CE Quevedo, V. Bavetsias, E. McDonald: Microwave-assisted synthesis of 2-aminonicotinic acids by reacting 2-chloronicotinic acid with amines . In: Tetrahedron Lett. tape 50 , no. 21 , 2009, p. 2481-2483 , doi : 10.1016 / j.tetlet.2009.03.034 .
- ↑ Patent US3415834 : Derivatives of 2-anilino nicotinic acid and process for their preparation. Applied December 15, 1964 , published December 10, 1968 , Applicant: Laboratoire UPSA, Inventor: C. Hoffmann, A. Faure.
- ↑ G. Cremonesi, L. Cavalieri: Efficacy and safety of morniflumate for the treatment of symptoms associated with soft tissue inflammation . In: J. Int. Med. Res. Band 43 , no. 3 , 2015, p. 290-302 , doi : 10.1177 / 030006054567212 .
- ↑ Patent EP0349902A2 : Processes for the preparation of morniflumate and analogous compounds. Applied on June 29, 1989 , published January 10, 1990 , Applicants: Chiesi Farmaceutici SpA, Inventors: P. Chiesi, V. Servadio, R. Pighi.
- ↑ Patent US5484931 : Process for preparing flunixin and intermediates thereof. Filed June 15, 1994 , published January 16, 1996 , Applicant: Schering Corp., Inventor: HJ Doran, DJ Coveney.
- ↑ Patent US4618366 : Certain N- (2,4-difluorophenyl) -2- (3-trifluoromethylphenoxy) nicotinamides having herbicidal activity. Applied June 15, 1984 , published October 21, 1986 , Applicant: May & Baker Ltd., Inventor: MC Cramp, J. Gilmour, EW Parnell.
- ↑ Patent EP0232067B2 : Substituted pyridinesulfonamide compounds, herbicidal composition containing them, and method of preparing these compounds. Applied January 21, 1987 , published March 16, 1994 , Applicants: Ishihara Sangkyo Kaisha Ltd., Inventors: F. Kimura, T. Haga, N. Sakashita, C. Honda, S. Murai.
- ↑ Patent US5366972 : 5,11-Dihydro-6H-dipyrido (3,2-B: 2 ', 3'-E) (1,4) diazepines and their use in the prevention or treatment of HIV infection. Filed on July 13, 1993 , published on November 22, 1994 , Applicant: Boehringer Ingelheim Pharmaceuticals, Inc., inventors KD Hargraves et al
- ↑ Patent US6680383B1 : Method for making nevirapine. Filed June 5, 2003 , published January 20, 2004 , Applicant: Boehringer Ingelheim Chemicals, Inc., Inventors: RF Boswell, BF Gupton, YS Lo.
- ↑ Patent WO2016118586A1 : Low cost, high yield synthesis of nevirapine. Filed January 20, 2016 , published July 28, 2016 , Applicant: Virginia Commonwealth University, Inventor: S. Ahmad, F. Gupton, J. Vergheses, T. McQuade.
- ↑ Patent WO199733846 : Process for the production of nitrobiphenyls. Registered on March 6, 1997 , published on September 18, 1999 , applicant: BASF AG, inventors: K. Eicken, J. Gebhardt, H. Rang, M. Rack, P. Schäfer.
- ↑ Patent US7241896B2 : Method for producing 2-halogen-pyridine-carboxylic acid amides. Registered on October 31, 2002 , published on July 10, 2007 , applicant: BASF AG, inventor: H. Mayer, D. Golsch, H. Isak, J. Schröder.
- ↑ LJ Goossen, N. Rodriguez: Decarboxylative Biaryl Synthesis from Carboxylic Acids and Aryl Halides . Science Forum Chemistry Ulm 2007 ( Online [PDF]).