3-dimethylaminoacrolein
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-dimethylaminoacrolein | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 9 NO | |||||||||||||||
| Brief description |
clear, pale yellow to dark brown liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 99.13 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
Easily soluble in water, in methanol and in 1,2-dichloroethane |
|||||||||||||||
| Refractive index |
1.584 - 1.588 at 20 ° C (589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-Dimethylaminoacrolein is a stable and significantly less toxic precursor for the genotoxic , mutagenic and potentially carcinogenic malondialdehyde in humans . The compound can be regarded as vinylogous dimethylformamide (DMF) and combines the functionalities of an unsaturated aldehyde and an enamine . Therefore, 3-dimethylaminoacrolein and the vinamidines derived therefrom (drawn together from vinylogous amidines ) or vinamidinium salts (substituted 1,5-diazapentadienes) are suitable as reactive molecular building blocks, especially for the construction of nitrogen-containing heterocycles , such as. B. of pyridines , pyrimidines , pyrroles or pyrazoles .
Occurrence and representation
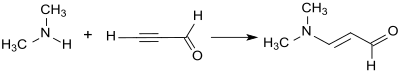
The addition of dimethylamine to the triple bond of propargylaldehyde in the sense of a vinylation according to Reppe produces 3-dimethylaminoacrolein in 88% yield as a yellow oil.
Because of its tendency to explode, propargylaldehyde is an unsuitable starting material for industrial syntheses of 3- (dimethylamino) -2-propenal.
Are more suitable vinyl ethers such. B. ethyl vinyl ether , which react with the Vilsmeier reagent formed from dimethylformamide (DMF) and phosgene in 68% yield to form 3-ethoxypropenylidene-dimethylammonium chloride, an enol ether iminium salt . In a weakly alkaline environment, 3-dimethylaminoacrolein is formed from it, which when exposed to strong bases, such as. B. sodium hydroxide solution , dimethylamine splits off with the formation of malondialdehyde .
With isobutyl vinyl ether , DMF and phosgene, which are easier to handle , higher yields (> 80%) of the iminium salt are achieved with continuous process management, from which 3-dimethylaminoacrolein is obtained in 86% yield with dilute sodium hydroxide solution.
Instead of phosgene, the iminium salt can also be mixed with an inorganic acid chloride , such as. B. phosphorus oxychloride or an organic acid chloride , such as. B. oxalyl chloride .
properties
3-Dimethylaminoacrolein is a clear, light yellow and water-soluble liquid that reacts slightly alkaline and with iron (III) chloride gives a deep red color. The compound "causes the hypnotic effect of morphine to be eliminated in mice" and has a "stimulating effect on humans".
use
Reactions with 3-dimethylaminoacrolein
3-Dimethylaminoacrolein can be used to introduce unsaturated and reactive C 3 groups into CH-acidic and nucleophilic compounds.
The activated aldehyde group of 3-dimethylaminoacrolein reacts quantitatively with dialkyl sulfates, such as. B. dimethyl sulfate with the formation of reactive but unstable adducts, which disintegrate again at 110 ° C into the starting materials. The adducts can easily be converted with nucleophiles such as alcoholates or amines to give the corresponding vinylogous amide acetals or amidines .
By reaction with sodium methoxide yield the stable 3-dimethylaminoacrolein dimethyl acetal that is generated in 62% acidic CH compounds, such. B. malonitrile to 1,3-butadiene derivatives or reacts with cyclopentadiene to form an aminofulvene .
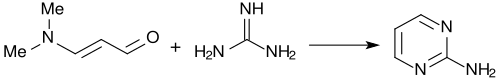
With guanidine , 3-dimethylaminoacrolein almost quantitatively forms 2-aminopyrimidine .
The amidine formed with 2-naphthylamine and the dimethyl sulfate adduct can be cyclized with sodium methoxide to give benzo [ f ] quinoline (1-azaphenanthrene).
N -Methylpyrrole forms 3- (2-N-methylpyrrole) propenal with 3-dimethylaminoacrolein and POCl 3 in 49% yield.
Analog manufacturing runs an intermediate stage of the cholesterol-lowering fluvastatin in the reaction of fluorarylsubstituierten N -isopropyl indole with 3-dimethylaminoacrolein and POCl 3 .
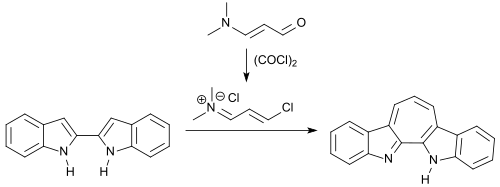
In the reaction with 3-dimethylaminocrolein and oxalyl chloride, 2,2'-bisindoles can be bridged by the 1-chloro-3- ( N , N -dimethylamino ) propenium chloride formed as an intermediate (as in the previous example) to form a seven-membered ring structure.
Occasionally the iminium salt from the reaction of the Vilsmeier reagent and the vinyl ether is used as a precursor of 3-dimethylaminoacrolein directly for synthesis, e.g. B. used by pyrazoles .
When using hydrazine hydrate , the base body pyrazole is produced in 84% yield.
Reactions to vinamidinium salts
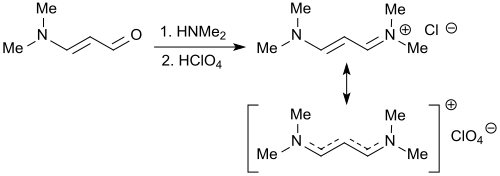
The reaction of 3-dimethylaminoacrolein with dimethylammonium tetrafluoroborate produces practically quantitatively the vinamidinium salt 3-dimethylaminoacrolein dimethyliminium tetrafluoroborate, which crystallizes better as a perchlorate salt and also reacts with cyclopentadiene in the presence of sodium amide in liquid ammonia to form the aminofulvene derivative.
The same vinamidinium salt 1,1,5,5-tetramethyl-1,5-diazapentadienium chloride is also formed in the reaction of 3-dimethylaminoacrolein with dimethylamine hydrochloride in 70% yield.
The action of dimethylamine and 70% perchloric acid on 3-dimethylaminoacrolein, which takes place in two stages , also forms the iminium salt referred to here as 1,3-bis (dimethylamino) trimethinium perchlorate.
Lactones , e.g. B. γ-butyrolactone or cyclic ketones , such as. B. Cyclopentanone and the vinamidinium salt of 3-dimethylaminoacrolein and dimethylamine hydrochloride form the corresponding dienaminones in 91% or 88% yield.
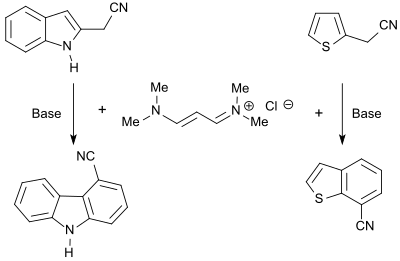
The vinamidinium salt 1,1,5,5-tetramethyl-1,5-diazapentadienium chloride reacts with heterocycles that carry CH-acidic groups, with the formation of the corresponding dienamines, which with bases to form fused heteroaromatics, such as. B. carbazoles , benzofurans or benzothiophenes can be cyclized.
N- alkylpyrroles are obtained in good yield (86%) in the reaction of the vinamidinium salt with glycine esters , substituted thiophenes (up to 87%) in the reaction with mercaptoacetic acid esters .
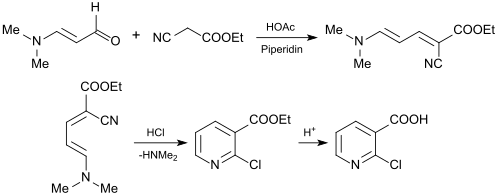
The use of 3-dimethylaminoacrolein for the synthesis of 2-chloronicotinic acid (2-CNA) as an important starting material for active pharmaceutical ingredients is of industrial interest . For this purpose, 3-dimethylaminoacrolein is reacted with ethyl cyanoacetate to give ethyl 2-chloronicotinate or with n-butyl cyanoacetate to give n-butyl 2-chloronicotinate in a Knoevenagel reaction .
The resulting esters of 2-chloropyridinecarboxylic acid can be hydrolyzed smoothly to give 2-chloronicotinic acid.
Individual evidence
- ↑ a b c d Entry on 3- (Dimethylamino) acrolein at TCI Europe, accessed on June 15, 2017.
- ↑ a b c d Data sheet 3- (Dimethylamino) acrolein 90% from Sigma-Aldrich , accessed on June 15, 2017 ( PDF ).
- ↑ a b c d Patent DE944852 : Process for the preparation of derivatives of 3-amino-acrolein. Registered on August 25, 1955 , published on June 28, 1956 , applicant: Badische Anilin- & Soda-Fabrik AG, inventor: F. Wille.
- ↑ a b c Patent DE2424373 : Process for the preparation of derivatives of malondialdehyde. Registered on May 20, 1975 , published on December 11, 1975 , applicant: BASF AG, inventor: M. Decker, W. Schönleben, H. Toussaint, H. Hoffmann.
- ↑ Patent US5780622 : Methods of synthesizing 5,15-diarylbenzochlorine-7-ones. Filed August 11, 1997 , published July 14, 1998 , Applicant: The University of British Columbia, Inventor: D. Dolphin, R. Boyle.
- ↑ LJ Niederhofer, JS Daniels, CA Rouzer, RE Greene, LJ Marnett: Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells . In: J. Biol. Chem. Volume 278 , 2003, p. 31426-31433 , doi : 10.1074 / jbc.M212549200 .
- ^ A b D. Lloyd, H. McNab: Vinamidine and Vinamidinium-Salze - Examples of stabilized push-pull alkenes . In: Angew. Chem. Band 88 , no. 15 , 1976, p. 496-504 , doi : 10.1002 / anie.19760881503 .
- ↑ S. Makhseed, HME Hassaneen, MH Elnagdi: Studies with 2- (Arylhydrazono) aldehydes: Synthesis and Chemical Reactivity of Mesoxalaldehyde 2-Arylhydrazones and of Ethyl 2-Arylhydrazono-3-oxopropionates . In: Z. Naturforsch. 62b, 2007, p. 529-536 ( znaturforsch.com [PDF]).
- ↑ P. Perlmutter: Propargyl Aldehyde . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rp262m .
- ↑ Z. Arnold, F. Sorm: Synthetic Reactions of dimethylformamide. I. General synthesis of β-dialdehydes . In: Collect. Czech. Chem. Commun. tape 23 , no. 3 , 1958, pp. 452-461 , doi : 10.1135 / cccc19580452 .
- ↑ Patent DE19825200C1 : Process for the production of 3-aminoacrolein derivatives. Registered on June 5, 1998 , published on November 18, 1999 , applicant: BASF AG, inventor: D. Golsch, M. Keil, H. Isak.
- ↑ H. Bredereck, F. Effenberger, G. Simchen: acid amide reactions, XXXII. About acid amide dialkyl sulfate complexes . In: Chem. Ber. tape 96 , no. 5 , 1963, pp. 1350-1355 , doi : 10.1002 / cber.19630960526 .
- ↑ H. Bredereck, F. Effenberger, D. Zeyfang: Synthesis and reactions of vinylogous amide acetals and amidines . In: Angew. Chem. Band 77 , no. 5 , 1965, pp. 219 , doi : 10.1002 / anie.19650770511 .
- ↑ C. Jutz, C. Jutz, RM Wagner: The synchronous six-electron cyclization of hexatriene systems as a new synthesis principle for the preparation of aromatics and heteroaromatics . In: Angew. Chem. Band 84 , no. 7 , 1972, p. 299-302 , doi : 10.1002 / anie.19720840714 .
- ↑ FW Ulrich, E. Breitmeier: Vinyloge Vilsmeier formylation with 3- (N, N-dimethylamino) -acrolinen . In: Synthesis . tape 8 , 1983, p. 641-645 , doi : 10.1055 / s-1983-30457 .
- ↑ D. Sriram, P. Yogeeswari: Medicinal Chemistry . 2nd Edition. Pearson, Delhi 2010, ISBN 978-81-317-3144-4 , pp. 364 .
- ↑ JT Zacharia, T. Tanaka, M. Hagashi: Facile and highly enenatioselective synthesis of (+) - and (-) - fluvastatin and their analogues . In: J. Org. Chem. Band 75 , no. 22 , 2010, p. 7514-7518 , doi : 10.1021 / jo101542y .
- ↑ Y. Kumai, R. Miyatake, Y. Sugeno, A. Ohta, M. Oda: Synthesis and spectroscopic properties of 1H-cyclohepta [2,1-b: 3,4-b '] diindole and molecular structure of its protonated species . In: Amer. J. Org. Chem. Volume 5 , no. 1 , 2015, p. 10-13 , doi : 10.5923 / j.ajoc.20150501.02 .
- ↑ Patent EP0731094A1 : Process for the production of pyrazoles. Registered on February 23, 1996 , published on September 11, 1996 , applicant: Bayer AG, inventor: H.-J. Wroblowsky, R. Lantzsch.
- ↑ Z. Arnold, J. Zemlicka: reactions of formamidinium salts and their vinylogs with carbanions . In: Collect. Czech. Chem. Commun. tape 25 , no. 5 , 1960, pp. 1302-1307 , doi : 10.1135 / cccc19601302 .
- ^ V. Nair, CS Cooper: Chemistry of 1,5-diazapentadienium (vinamidinium) salts: alkylation reactions to multifunctional dienamines and dienaminones . In: J. Org. Chem. Band 46 , no. 23 , 1981, pp. 4759-4765 , doi : 10.1021 / jo00336a027 .
- ^ Z. Arnold, D. Dvorak, M. Havranek: Convenient preparation of 1,3-bis (dimethylamino) trimethinium perchlorate, tetrafluoroborate and hexafluorophosphate . In: Collect. Czech. Chem. Commun. tape 61 , no. 11 , 1996, pp. 1637-1641 , doi : 10.1135 / cccc19961637 .
- ^ V. Nair, CS Cooper: Selective alkylation reactions with vinamidinium salts . In: Tetrahedron Lett. tape 21 , no. 33 , 1980, pp. 3155-3158 , doi : 10.1016 / S0040-4039 (00) 77433-8 .
- ↑ MT Wright, DG Carroll, TM Smith, SQ Smith: Synthesis of alkyl pyrroles by use of a vinamidinium salt . In: Tetrahedron Lett. tape 51 , no. 31 , 2010, p. 4150-4152 , doi : 10.1016 / j.tetlet.2010.06.009 .
- ^ RT Clemens, SQ Smith: The application of vinamidinium salts to the synthesis of 2,4-disubstituted thiophenes . In: Tetrahedron Lett. tape 46 , no. 8 , 2005, p. 1319-1320 , doi : 10.1016 / j.tetlet.2004.12.113 .
- ↑ Patent EP0372654A2 : Preparation of 2-chloropyridine 3-carboxylic acid esters. Applied on December 5, 1989 , published on June 13, 1990 , applicant: Shell Internationale Research Maatschappij BV, inventor: L. Schröder.
- ↑ Patent WO0007989A1 : Process for the production of 2-halo nicotinic acid derivatives and 2-halo nicotinic acid n-butyl ester as an intermediate. Registered on July 14, 1999 , published on February 17, 2000 , applicant: BASF AG, inventor: D. Golsch, M. Keil, H. Isak, H. Mayer.




![Synthesis of benzo [f] quinoline with 3-dimethylaminoacrolein](https://upload.wikimedia.org/wikipedia/commons/thumb/6/68/Synthese_von_1-Azaphenanthren.svg/400px-Synthese_von_1-Azaphenanthren.svg.png)