Amyl nitrite
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Amyl nitrite | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 5 H 11 NO 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 117.15 g · mol -1 | |||||||||||||||||||||
| density |
0.87 g cm −3 (20 ° C) |
|||||||||||||||||||||
| boiling point |
97-99 ° C |
|||||||||||||||||||||
| Vapor pressure |
65 hPa (20 ° C) |
|||||||||||||||||||||
| Refractive index |
1.3918 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Amyl nitrite is a name for an ester of the aliphatic alcohol amyl alcohol with nitrous acid . However, the use of the term is often imprecise and not tied to a specific chemical structure.
Amyl nitrite chemically belongs to the group of alkyl esters of nitrous acid ( alkyl nitrites ) such as ethyl nitrite , propyl nitrite , isopropyl nitrite , 1-butyl nitrite or isobutyl nitrite .
Definition of terms
Amyl nitrite referred strictly speaking only of n pentanol outgoing n -Pentylester of nitrous acid . All other pentyl esters that come from the branched pentanols - especially (3-methylbutyl) nitrite - are chemically correctly referred to as iso- amyl nitrites, but isoamyl nitrite is often used as a group name that includes the n- pentyl ester. There is also synonymous use of “isoamyl nitrite” and “amyl nitrite”.
history
The French chemist Antoine-Jérôme Balard (1802–1876) produced amyl nitrite for the first time in 1844. In 1859 J. Guthrie observed that inhaling the amyl nitrite vapors accelerated the pulse and reddened the face. Benjamin Ward Richardson (1828–1896) tested the effect on frogs and found vasodilation of the mesenteric vessels . Arthur Graham Gamgee (1841-1893) was able to demonstrate an antihypertensive effect on mammals. Based on these findings, amyl nitrite was introduced into angina pectoris therapy in 1867 by the pharmacologist Thomas Lauder Brunton (1844–1916) . Corneille Heymans (1892–1968) did not explain the mechanism of action until 1931 .
Isomers and properties
| Structural isomers | ||||
| Surname | n -amyl nitrite | 3-methylbutyl nitrite | Isoamyl nitrite (mixture of isomers) | |
| other names |
n -Pentylnitrit |
3-methyl-1-nitrosooxybutane |
Isopentyl |
|
| Structural formula |
|
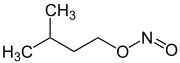
|
||
| CAS number | 463-04-7 | 110-46-3 | 8017-89-8 | |
| EC number | 207-332-7 | 203-770-8 | ||
| ECHA info card | 100.006.667 | 100.003.429 | ||
| PubChem | 10026 | 8053 | 24687 | |
| ChemSpider | 9632 | 7762 | ||
| ZVG number | 510045 | 496177 | ||
| Wikidata | Q412350 | Q27888090 | Q54086381 | |
3-methylbutyl nitrite is a pale yellow, easily mobile liquid with a density of 0.87 g · cm −3 (20 ° C) and a boiling point of 98–99 ° C. The compound is infinitely miscible with most organic solvents, but is not very soluble in water, but is easily saponified (especially in the presence of bases or acids). It has a characteristic, sweetish, dull odor.
Effect and use
Amyl nitrite was originally used medically for the temporary expansion of the coronary arteries in angina pectoris and to lower blood pressure. Because of its short duration of action, diluted nitroglycerin (trade name Nitrolingual ) is used today. An induced by amyl nitrite methemoglobinemia helps in the treatment of cyanide - poisoning because the methemoglobin while verträglicherem cyanomethemoglobin responding; today, however, this method is no longer the method of choice.
Due to a psychotropic and aphrodisiac effect that sets in quickly after inhalation , amyl nitrite is misused in the form of so-called " poppers " ( see section on legal position ).
Organic nitrites are NO donors. In the endothelial cells of blood vessels, nitric oxide NO has a muscle-relaxing and thus vasodilating effect through second messenger mechanisms . Since the venous blood vessels react more strongly to NO donors due to better enzymatic equipment, there is initially increased blood flow and improved oxygen supply in the normal dose range ( reddening of the face, medical " flush "). Overdosing can lead to an acute drop in blood pressure, in extreme cases to shock , if the blood supply to the brain can no longer be guaranteed due to the dilation of blood vessels in the body.
Mutagenic , toxic or immunosuppressive properties have not been demonstrated. Drugs containing nitrite are generally only available on prescription. With low blood pressure, the use of amyl nitrite is contraindicated. Contact of liquid amyl nitrite with mucous membranes and eyes can cause irritation and chemical burns.
In preparative organic chemistry, amyl nitrite is used as a nitrosating agent. A distinction must be made between this and amyl nitrate , which, like other nitrates, is used, for example, as an ignition accelerator for diesel fuels.
Contraindication
Simultaneous use of medication containing nitrates such as nitrolingual spray or NO donors with PDE-5 inhibitors (e.g. sildenafil , tadalafil or vardenafil ) is contraindicated . The combined effect on blood pressure threatens an acute, life-threatening drop in blood pressure.
Side effects
Adverse effects can be dizziness, drowsiness, palpitations, drop in blood pressure up to life-threatening circulatory collapse, impaired articulation, headache, nausea, disorientation, reddening of the skin and hot flashes. It can lead to increases in intracranial pressure and intraocular pressure. Yellow-colored visual perception is also reported immediately after consumption.
Countermeasures in the event of overdose
Symptom-oriented emergency treatment because of the cardiovascular-related symptoms: moving the extremities, keeping the head low, inhaling and exhaling deeply (aim: promoting venous return). Adrenaline is not the drug of choice because it aggravates the symptoms of shock (weakness, restlessness, sweating, paleness, nausea and vomiting, urinary and fecal incontinence ).
With regard to possible dyspnea (shortness of breath), the antidote methylene blue as an injection is the drug of choice, as this accelerates the breakdown of methemoglobin , which causes dyspnea and which results in nitrite intoxication. With simultaneous treatment of Cyanidintoxikation prior to administration of methylene blue is an iatrogenic methemoglobinemia , cyanide metabolism to deal turn dangerous, must be observed.
Addiction
There is no known physical addiction; the development of a psychological addiction is considered possible and should be expressed in reluctance to have sex without amyl nitrite.
Legal position
Amyl nitrite is not subject to any narcotics regulations, so possession is legal. However, manufacturing and placing on the market without permission for use on humans or animals are prohibited in many countries, since amyl nitrite, as a pharmacologically active substance, falls under the definition of pharmaceutical regulations (in the EU, for example, Directives 2001/82 / EC and 2001/83 / EC ) and is regulated by such.
Individual evidence
- ↑ a b c d e data sheet isopentyl nitrite (PDF) from Merck , accessed on May 24, 2018.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-312.
- ↑ a b c Entry on 3-methylbutyl nitrite in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Pentyl nitrite in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Barbara I. Tshisuaka: Brunton, Sir Thomas Lauder. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 217.
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 161 .
- ↑ Entry on n-pentyl nitrite in the ChemSpider database of the Royal Society of Chemistry , accessed on May 20, 2018.
- ↑ Entry on Iso-Amyl nitrite in the ChemSpider database of the Royal Society of Chemistry , accessed on May 20, 2018.
- ↑ entry to pentyl in GESTIS Bank of IFA , accessed on 2018-05-20(JavaScript required) .
- ↑ Article 1 No. 2 (b) of Directives 2001/82 / EC and 2001/83 / EC, implemented in Germany in Section 2, Paragraph 1 of the Medicines Act .
- ↑ For Germany see also: Erwin Deutsch, Rudolf Ratzel, Hans-Dieter Lippert: Commentary on the Medicines Act (AMG). 3rd edition, Gabler Wissenschaftsverlage, 2010, ISBN 978-3-6420-1454-3 , pp. 64–66 ( limited preview in the Google book search).
