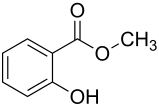
Salicylic acid methyl ester
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Salicylic acid methyl ester | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 8 H 8 O 3 | ||||||||||||||||||
| Brief description |
|
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 152.14 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.18 g cm −3 |
||||||||||||||||||
| Melting point |
−8 ° C |
||||||||||||||||||
| boiling point |
223 ° C |
||||||||||||||||||
| Vapor pressure |
13 Pa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.535 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Salicylic acid methyl ester , also methyl salicylate , is the methyl ester of salicylic acid . Salicylic acid methyl ester is also known as wintergreen oil or Gaultheria oil , as it can be isolated from the leaves of the genera Pyrola ( wintergreen ) and Gaultheria ( pseudo-berries ) by means of steam distillation .
Occurrence
Plants that produce large (that is, smellable) amounts of methyl salicylate include:
- many species of the heather family , especially the varieties of the lower shamberry (wintergreen shrubs) and the real meadowsweet ( Filipendula ulmaria ), where the active ingredient occurs in the roots.
- some types of birch , especially the sugar birch , but also the yellow birch .
The sugar birch used to be the main source of methyl salicylate. The product made from the leaves of the wintergreen bushes is known as Wintergreen Oil .
Manufacturing
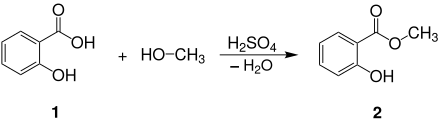
Salicylic acid methyl ester 2 is obtained by esterification of salicylic acid 1 with methanol , sulfuric acid being used as a catalyst :
use
Salicylic acid methyl ester in the synthetic form is used externally in ointments and other liniments as a blood circulation-promoting (hyperaemic) agent, for example for rheumatic complaints . It is also used for this purpose in the form of bath additives. It is preferably used in its natural form as wintergreen oil for cosmetics and oral care products, and also in perfumery and the food industry, as it has a very characteristic odor.
The essential oil of wintergreen , which mainly contains methyl salicylate, is still used in microscopy for lightening preparations according to Spalteholz .
In North America, almost all chewing gum manufacturers offer the classic flavors such as peppermint as well as the winter green flavor , which contains methyl salicylate. In Europe, this flavor has not yet been able to establish itself in chewing gum; many Europeans find the taste clinically dentist-like and not tasty. The flavor is also often offered in snus in America and Scandinavia.
The products with a wintergreen aroma that are still most widely known in Germany are the lollipop brand Tic Tac from the Italian confectionery manufacturer Ferrero and the chewing gum brand Bazooka from the US manufacturer Topps Company , which was licensed by the German confectionery manufacturer August Storck until the end of the 1980s was produced and distributed. Nowadays, Wintergreen chewing gum and pastilles are only available in Germany as imported goods, for example in American outlet stores.
Because of the intense smell, attempts were made at the paint factories vorm. Friedr. Bayer & Co. to offer methoxymethyl salicylate as an alternative. It was presented by Arthur Eichengrün in the Pharmazeutische Zeitung of 1902 under the trade name Mesotan .
Side effects and restrictions on use
Salicylic acid methyl ester should, if possible, not be used during pregnancy , as insufficient data are available. If commercially available preparations are used on the skin, no toxic effects are generally to be expected, since serum levels above 50 µg / ml are rarely reached. In the event of improper use such as long-term or extensive application or application to non-intact skin, side effects such as dizziness, ringing in the ears, nausea or other gastrointestinal complaints and an influence on the blood coagulation system cannot be ruled out because of a possible systemic salicylate effect . In the United States, a female athlete is said to have died of an overdose of the active ingredient.
Botanical background
Salicylic acid methyl ester can be toxic, especially if it is ingested. Plants containing methyl salicylate may have developed it as a deterrent against predators. Salicylic acid methyl ester can also be used by plants as a pheromone to warn other plants about pathogens such as the tobacco mosaic virus . If the plant is infested with insects, salicylic acid methyl ester can act as an attractant for beneficial insects which kill the attacking insects. Many plants produce small amounts of methyl salicylate.
Chemical properties
Salicylic acid methyl ester can be nitrated with iron (III) nitrate . An isomer mixture is formed from the 3- and 5-nitro derivatives. In the presence of nitric acid , mainly 5-nitrosalicylic acid methyl ester 4 is formed , in a basic medium mainly 3-nitrosalicylic acid methyl ester 3 .
The acetylation of methyl salicylate with acetic anhydride in the presence of aluminum chloride as a catalyst takes place with very good yields at the C5 carbon.
Through the reaction with sodium hypochlorite , up to two chlorine atoms can be introduced into the benzene nucleus, resulting in methyl 3,5-dichlorosalicylate.
In the reaction with elemental bromine, a bromine atom is added to the 5-position.
Risk assessment
In 2013, salicylic acid methyl ester was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of methyl salicylate were concerns regarding consumer use and high (aggregated) tonnage as well as the dangers arising from a possible assignment to the group of CMR substances. The re-evaluation has been running since 2015 and is carried out by France . In order to be able to reach a final assessment, further information was requested.
further reading
- TY Chan: The risk of severe salicylate poisoning following the ingestion of topical medicaments or aspirin. In: Postgraduate medical journal. Volume 72, Number 844, February 1996, pp. 109-112, ISSN 0032-5473 , PMID 8871462 . PMC 2398362 (free full text).
Individual evidence
- ↑ Entry on METHYL SALICYLATE in the CosIng database of the EU Commission, accessed on December 28, 2019.
- ↑ a b Entry on salicylic acid esters. In: Römpp Online . Georg Thieme Verlag, accessed on February 1, 2012.
- ↑ a b European Pharmacopoeia, 8th edition, Grundwerk 2014, p. 4097.
- ↑ a b c d e f g Entry on methyl salicylate in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-376.
- ↑ Entry on salicylic acid methyl ester in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b R. Hansel, O. Sticher: Pharmakognosie - Phytopharmazie . 9th edition, Wissenschaftliche Verlagsges.mbH, Stuttgart 2010, p. 1036.
- ↑ RÖMPP Lexikon Chemie, 10th edition, 1996–1999: Volume 6: T - Z.
- ↑ Patent DE137585 : Process for the preparation of the alkoxymethyl esters of salicylic acid. Published on November 26, 1902 , applicant: Farbenfabriken, vorm. Friedr. Bayer & Co. in Elberfeld.
- ↑ Patent US740628 : Alkoxyalkylidene Esters of Salicylic Acid. Published on October 6, 1903 , inventor: Jürgen Callsen.
- ↑ Patent US706018 : Alkyloxymethyl Esters of Salicylic Acid and Process of Making Same. Published on August 5, 1902 , inventor: Jürgen Callsen.
- ↑ A. Eichengrün: About Aristochin, Mesotan, Helmitol and Theoein . In: Pharmaceutical newspaper . tape 47 , November 1902, p. 857–858 , urn : nbn: de: gbv: 084-11022408557 .
- ↑ A. Eichengrün: About Aristochin, Mesotan, Helmitol and Theoein (conclusion) . In: Pharmaceutical newspaper . tape 47 , November 1902, p. 866–867 , urn : nbn: de: gbv: 084-11022408557 .
- ↑ Specialist information Trauma Ointment cooling Mayrhofer , Kwizda Pharma GmbH, Vienna. Status: March 2018
- ↑ Better labels urged for sports creams , USA Today , The Associated Press, June 2007.
- ↑ Y.-Z. Liu, X.-Y. Li, X. Li, L. Zhang: Nitration Reaction between Methyl Salicylate and Iron (III) Nitrate and Its Regioselectivity. In: Chinese Journal of Applied Chemistry. 2010 . Abstract .
- ↑ Pia Kahnberg, Choon Woo Lee, Robert H Grubbs, Olov Sterner: Alternative routes to pterulone . In: Tetrahedron . tape 58 , no. June 26 , 2002, p. 5203-5208 , doi : 10.1016 / S0040-4020 (02) 00505-7 .
- ↑ WB Salter, JR Owens, JD Wander: Methyl Salicylate: A Reactive Chemical Warfare Agent Surrogate to Detect Reaction with Hypochlorite. In: ACS Appl. Mater. Interfaces. 2011 , 3 (11), pp. 4262-4267 doi: 10.1021 / am200929v .
- ^ OS Tee, NR Iyengar: The bromination of salicylate anions. Evidence for the participation of the ortho carboxylate group. In: J. Org. Chem. 1985 , 50 (23), pp. 4468-4473. doi: 10.1021 / jo00223a011 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Methyl salicylate , accessed on March 26, 2019.