Milarite group
The Milaritgruppe (Osumilithgruppe, Milarit-Osumilith-Gruppe) is a group of minerals from the division of ring silicates , which all have the same structural structure . Their composition obeys the general formula:
CB 2 A 2 [T2 3 T1 12 O 30 ].
In this structural formula , the capital letters A, B, C, T1 and T2 represent different positions in the milarite structure, with
- C: K, Na, Ba, □ (space)
- B: □, H 2 O, Na, K,
- A: Ca, Mg, Fe 2+ , Fe 3+ , Mn 2+ , Mn 3+ , Ti, Sn, Zr, Y, Yb, Sc, Na, Nb, Ta
- T2: Li, Be, Mg, Zn, Fe 2+ , Fe 3+ , Al, B
- T1: Si, Al
In their optical and physical properties, the minerals of the milarite group are very similar to other minerals such as quartz , apatite , beryl or cordierite , with which they can occur together, and are therefore often overlooked.
They form six-sided prismatic to plate-like crystals or inconspicuous, small crystals or granular aggregates. Depending on the composition, they are colorless, pale pink, purple, blue, green, or brown with a glassy to greasy sheen . They show clear to good cleavage perpendicular to the prismatic surfaces and are quite hard with a Mohs hardness of 5–6 (2.5–3: Berezansite, up to 7.5: armenite).
With a few exceptions (e.g. milarite, osumilite), the individual minerals in this group are very rare, but generally distributed worldwide. They occur mainly in rocks that are formed in the late phase of igneous intrusions : pegmatites and hydrothermal veins .
Etymology and history
From zeolite to ring silicate: milarite and armenite
The history of the Milaritgruppe begins with a few mistakes - and it owes its name to the first. The Swiss mineral trader GR Köhler acquired some mineral specimens in Tavetsch ( Graubünden , Switzerland ) in the 1860s, the place of which he was told was Val Milar. The mineralogist Gustav Adolf Kenngott "soon recognized it for something new" , acquired the pieces in 1869 and named the mineral he described after the alleged place of discovery - milarite. Giuvit after the neighboring valley Val Giuv would have been correct.
In his letter of September 30, 1869 to Gustav von Leonhard , professor at the University of Heidelberg and editor of the New Yearbook for Mineralogy, Geology and Paleontology , he described milarite as a new zeolite . The "boiling stones", as zeolite is translated, currently fascinated the mineralogically interested society and from Scotland to Italy new zeolites were discovered and scientifically described. Only in Switzerland they were hardly found at that time, which is how Kenngott introduced his report. The assignment to the zeolite group is understandable against this background and with the analytical possibilities at that time, but not correct from today's perspective.
Around 60 years later, systematic studies of the beryllium content of a large number of minerals carried out at Harvard University showed that beryllium is an essential component of milarite.
The next discovery of a mineral from the milarite group was made in 1939 by Henrich Neumann at the University of Oslo in Norway . Not unusual for the often confused minerals of this group was the sample since 1877, collected by mineralogy student OA Corneliussen in the poor mine near Kongsberg and named " Epidote ?" labeled, in the collection of the Oslo Institute of Mineralogy. Neumann examined the finds again and named the new water-containing barium-calcium-aluminum silicate after the place where it was found: armenite.
In another publication two years later, he shows the relationship between armenite and milarite and questions the classification as zeolite - the dewatering temperature of 500 to 600 ° C is too high for zeolitic water. However, he omits a systematic classification with reference to a lack of X-ray structure analyzes.
The structure then cleared up NV Belov and TN Tarkova in 1949 and 1951. They were able to describe milarite as a double ring silicate with Be in another tetrahedral position and recognized the structural relationship with beryl .
From low to high formation temperatures: Osumilith
The minerals that Akiho Miyashiro of the University of Tokyo investigated in 1951 did not come from low-temperature, hydrothermal veins, such as milarite and armenite, but were clearly of magmatic origin. They came from the finely crystalline matrix and small cavities of a rhyodacite from the Ōsumi province , Japan , where they occur together with tridymite , which suggests quite high formation temperatures. Logically, they were initially mistaken for a long-known mineral - cordierite in this case or a high-temperature modification of the same. Upon closer examination of these unusual, optically uniaxial "Cordierites", Miyashiro was able to show that it was a new mineral from the milarite group and named it after its origin: Osumilite. He also expressed the suspicion that many of the “cordierites” that had been described in comparable igneous rocks in the 50 years before were also Osumilite.
From the beginning of the solar system: Merrihueit, Roedderit, Yagiit
The 1960s were the extraterrestrial decade of the milarite group. Until then, minerals with a milarite structure were only known from rocks that were the products of longer crystallization and fractionation processes, which resulted in the accumulation of alkali and alkaline earth metals such as Li, Na, K, Be, Ba or H 2 O, among others . Meteorites, on the other hand, are very primitive rocks that are at the very beginning of such fractionation processes. Nevertheless, numerous investigations into the mineral inventory of meteorites worldwide then led to the discovery of minerals with a milarite structure in this environment too.
In 1965 the first K- and Na-rich mineral in a meteorite was discovered in the Mezö-Madaras meteorite, which fell on September 4th, 1852 in Mezömadaras, Transylvania , Romania : Merrihueit . In contrast to all previously known minerals with a milarite structure, it does not contain any aluminum.
In 1966, in the Indarch meteorite, which fell on April 7, 1891 in Shusha, Autonomous Republic of Nakhichevan , Azerbaijan , the Mg analogue of Merrihueit was discovered with Roedderite .
In 1968 the investigation of the Colomera meteorite, an iron meteorite found in 1910 near Granada , Andalusia , in Spain , led to the discovery of the yagiite .
In 2006, the Stardust spacecraft brought material from comet 81P / Wild 2 to Earth that unexpectedly also contained high-temperature minerals such as roedderite .
In 2014, porous, chondritic micrometeorites were recovered from the Antarctic snow and ice that resemble samples of interplanetary dust particles collected by aircraft in the stratosphere . These dust particles represent the most pristine material from the time our solar system was formed and, in addition to enstatite and cosmochlor, also contained roedderite.
From different corners of the world: Eifel, Tørdal, Tajikistan ...
Almost as remote as meteorites the complex alkali richer Granitoids and their boron -rich and SiO 2 -untersättigter pegmatite in Alay Mountains in Tadschikistan . The moraines of the Darai-Pioz glacier are the type locality of 35 minerals, 5 of them from the milarite group:
- 1969: Sogdianite
- 1975: Darapiosit
- 1994/95: Dusmatovit
- 1996: Berezanskit
- 1998: Shibkovit
The last mineral of the milarite group to date, Agakhanovit- (Y), was discovered in 2014 in Heftetjern pegmatite, Tørdal , Norway . Only a few years earlier, in 2006, Oftedalite was described from there, a member of the milarite group containing scandium.
Occurrence
The high variability of the compositions is reflected in the diversity of the geological milieus in which minerals of the milarite group can be found. Most of them are alkali-rich magmatites , pegmatites and hydrothermal solutions or xenolites in syenitic breccias , where they crystallize at low pressures and temperatures often below 300 ° C, but also acidic magmatites (Osumilith), granulite-facial metamorphites (Osumilith), metamorphic manganese deposits (Sugilite) and Meteorites (Yagiit, Roedderit, Merrihueit).
This very large stability range of minerals of the milarite group is often countered by only a few documented occurrences of the individual minerals. This is attributed to the fact that these minerals can easily be confused with common minerals of the same occurrence, e.g. B. with quartz , apatite , cordierite or beryl . Many minerals of this group have only been discovered in the last 30 years and it is assumed that the minerals of this group are much more common than previously known.
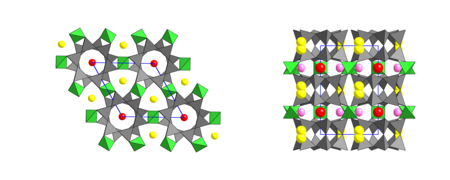
Crystal structure
The minerals of the milarite group are mostly hexagonal with the space group P 6 / mcc (space group no.192 ) ( P 6 / mmm ( no.191 ) : Almarudite) with the lattice parameters a = 9.903 Å (Berezanskit) to 10.505 Å (Shibkovit) and c = 13.48 Å (Poudretteite) to 14.39 Å (Darapiosit) and two formula units per unit cell .
Ordered incorporation of Al into the silicate double rings and H 2 O in the B-position lead to reduced orthorhombic symmetry in armenite with the space group Pnna (space group no. 52) and 4 formula units per unit cell .
T1: silicate anion
The T1 position is tetrahedrally surrounded by 4 oxygen anions. The cation, predominantly Si 4+ , sits in the center and the oxygen anions on the corners of a tetrahedron. Six of these tetrahedra have common corners, i.e. H. Oxygen ions, linked to form rings and two of these rings in turn to double rings via corners. These six-double-ring silicate anions are not direct; H. Connected to one another via Si-O-Si bonds, and the minerals of the milarite group are therefore counted among the ring silicates in the classifications of Dana and Strunz.
T2: framework structure
The T2 position is also tetrahedrally surrounded by 4 oxygen anions. It lies between the T1-6 double rings and is connected to each tetrahedron with a T1 position. Each T2 tetrahedron connects four T1 double rings via common corners to form a four-fold, three-dimensional TO 2 framework. According to the Zoltai and Liebau systems, the milarite structure is one of the framework structures.
Theoretically, there is a whole series of framework structures of four-linked tetrahedra with double rings, of which only a few occur in mineral structures. The only other naturally occurring tetrahedral framework structure with tetrahedral double rings is that of steacyite with four double rings. Another framework structure closely related to the milarite, six-membered single rings, which are connected by further tetrahedra to form four-fold linked frameworks, can be found in the minerals of the beryl group and in cordierite .
C position
The C position, which is predominantly occupied by potassium ions (K + ), is surrounded by 12 oxygen ions. It lies between two double rings in the center of the rings and is very similar to the K position in mica .
B position
The position, which is often empty or partially occupied by water or Na + , is surrounded by 9 oxygen ions and, especially in the case of water-containing minerals of this group, is split into two sub-positions, which are superimposed along the c-axis and of which only one is occupied. The B position fills the cavity between three double rings at the level of the rings.
A position
The A position is surrounded by 6 oxygen ions in the form of a distorted octahedron . This position, like the B position, is split into two sub-positions along the c-axis. This position is also located in the channels running in the c direction between two B positions at the level of the T2 positions.
Depending on which cations are incorporated in this position, the distortion of the oxygen environment can be very different. If large, low-charge cations are incorporated here, such as B. Ca 2+ in milarite, the distortion of the coordination is high. If small, highly charged cations are incorporated, such as B. Fe 3+ in sugilite, the A position is very symmetrically surrounded by 6 oxygen atoms on the corners of an almost undistorted octahedron.
The neighboring T2 position behaves in the same way. If there are low-charge cations such as Li + in the sugilite, the T2 tetrahedron is extremely distorted, with highly charged cations such as Al 3+ the distortion is only slight.
The comparatively rigid silicate double rings are connected by the chemically variable and flexible positions A and T2. Their flexibility contributes significantly to the great stability of the milarite structure under the most varied of pressure and temperature conditions and enables a wide variety of composition.
Chemism
The large number of different minerals of the milarite group is essentially due to variations in the occupation of the T2 and A positions. The 12-fold coordinated C-position is almost always fully occupied with potassium and the variable contents of vacancies and Na ions in the B-position compensate for smaller charge deficits or excesses in other positions.
The following table shows the minerals of the milarite group grouped according to the cation charge on the T2 tetrahedron position. The right column gives the empirical compositions, as result from a normalization of the measured concentrations to 30 oxygen. Small deviations from the expected stoichiometry, such as B. slightly more than 12 Si atoms can occur.
The other columns show the idealized composition with an assignment to the cation positions.
| Surname | C. | B 2 | A 2 | T2 3 | T1 12 | composition |
|---|---|---|---|---|---|---|
| 3M + on T2 | ||||||
| Alumosugilite | K | Well 2 | Al 3+ 2 | Li 3 | Si 12 O 30 | |
| Sugilite | K | Well 2 | Fe 3+ 2 | Li 3 | Si 12 O 30 | K 1.001 Na 1.765 (Fe 3+ 1.124 Al 0.496 Mn 3+ 0.304 ) Li 3.034 Si 12 O 30 |
| hypothetical [A] Y 3+ [T2] Li end link | K | Well 2 | Y 3+ 2 | Li 3 | Si 12 O 30 | |
| synthetic [A] Cr 3+ [T2] Li end link | K | Well 2 | Cr 3+ 2 | Li 3 | Si 12 O 30 | |
| Berezanskit | K | □ 2 | Ti 4+ 2 | Li 3 | Si 12 O 30 | (K 0.98 Na 0.06 Ba 0.01 ) (Ti 1.94 Nb 0.04 Fe 0.02 ) (Li 2.95 Al 0.02 ) Si 11.99 O 30 |
| Brannockite | K | □ 2 | Sn 4+ 2 | Li 3 | Si 12 O 30 | (K, Na) Sn 4+ 2 Li 3 [Si 12 O 30 ] |
| Sogdianite | K | □ 2 | Zr 4+ 2 | Li 3 | Si 12 O 30 | (K 0.99 Na 0.01 ) (Zr 4+ 1.98 Hf 0.02 ) Li 2.97 Si 12 O 30 |
| M + 2M 2+ on T2 | ||||||
| Darapiosit | K | Well 2 | Mn 2+ 2 | Zn 2 Li | Si 12 O 30 | K (Na 1.22 K 0.36 □ 0.42 ) (Mn 2+ 1.54 Zr 0.30 Y 0.23 Mg 0.03 ) (Li 1.53 Zn 1.15 Fe 2+ 0.31 ) Si 11.98 O 30 |
| hypothetical [A] M 3+ Zn 2 Li end link | K | □ 2 | M 3+ 2 | Zn 2 Li | Si 12 O 30 | |
| Trisodium tetramagnesium lithium closo-30-oxododecasilicate (synthetic) | N / A | Well 2 | Mg 2+ 2 | Mg 2+ 2 Li | Si 12 O 30 | NaNa 2 Mg 2 (Mg 2 Li) Si 12 O 30 |
| Tripotassium tetramagnesium lithium closo-30-oxododecasilicate (synthetic) | K | K 2 | Mg 2+ 2 | Mg 2+ 2 Li | Si 12 O 30 | KK 2 Mg 2 (Mg 2 Li) Si 12 O 30 |
| 3M 2+ on T2 | ||||||
| Eifelit | K | Well 2 | MgNa | Mg 3 | Si 12 O 30 | (K 0.92 Na 0.08 ) (Na 1.82 □ 0.18 ) (Mg 1.20 Na 0.80 ) (Mg 2.78 Zn 0.05 Cu 0.02 Mn 2+ 0.08 Fe 2+ 0.03 Al 0.04 ) (Si 11.92 Al 0.08 ) O 30 |
| unnamed | K | K 2 | Mn 2+ 1.5 □ 0.5 | Zn 3 | Si 12 O 30 | K (K 0.58 Na 0.42 ) (Mn 2+ 1.5 □ 0.5 ) Zn 3 Si 12 O 30 |
| synthetic phase from glaze of a scarab | N / A | Well 2 | Mg 2 | Mg 3 | AlSi 11 O 30 | (K 0.12 Na 0.88 ) (Na 1.28 □ 0.72 ) Mg 2 (Mg 2.58 Cu 0.24 Fe 0.18 ) (Al 0.6 Si 11.4 ) O 30 |
| Roedderite | K | □ Well | Mg 2 | Mg 3 | Si 12 O 30 | (Na 1.30 K 0.69 ) (Mg 4.86 Fe 0.27 ) (Al 0.07 Si 11.88 ) O 30 |
| Merrihueit | K | □ Well | Fe 2+ 2 | Fe 2+ 3 | Si 12 O 30 | K 0.47 Na 0.38 Ca 0.06 Mn 0.08 Mg 1.27 Fe 3.85 Al 0.02 Si 12 O 30 |
| Disodium pentamagnesium closo-30-oxododecasilicate (synthetic) | N / A | □ Well | Mg 2+ 2 | Mg 2+ 3 | Si 12 O 30 | (Na 0.6 □ 0.4 ) (Na 1.4 □ 0.6 ) Mg 2 Mg 3 Si 12 O 30 |
| Rubidium sodium pentamagnesium dodecasilicate (synthetic) | Rb | □ Well | Mg 2+ 2 | Mg 2+ 3 | Si 12 O 30 | RbNaMg 2 Mg 3 Si 12 O 30 |
| synthetic Mn milarite | K | □ Well | Mn 2+ 2 | Mn 2+ 3 | Si 12 O 30 | |
| Disodium trimagnesium dicopper closo-30-oxododecasilicate (synthetic) | □ | Well 2 | Mg 2+ 2 | Mg 2+ Cu 2 | Si 12 O 30 | (□ 0.6 Na 0.4 ) (Na 1.6 □ 0.4 ) (Mg 1.85 Cu 0.15 ) (Mg 1.15 Cu 1.85 ) Si 12 O 30 |
| Dipotassium trimagnesium dicopper closo-30-oxododecasilicate (synthetic) | K | K □ | Mg 2+ 2 | Mg 2+ Cu 2 | Si 12 O 30 | K (K □) (Mg 1.75 Cu 0.25 ) (Mg 1.25 Cu 1.75 ) Si 12 O 30 |
| Dusmatovite | K | □ Well | Mn 2+ 2 | Zn 3 | Si 12 O 30 | K (Na 0.64 K 0.36 □) (Mn 1.52 Zr 0.24 Y 0.24 ) (Zn 2.28 Li 0.72 ) Si 12 O 30 |
| Shibkovite | K | □ K | Approx 2 | Zn 3 | Si 12 O 30 | K (K 1.20 □ 0.80 ) (Ca 1.26 Mn 0.40 Na 0.39 Fe 0.01 ) Zn 3.01 (Si 12.01 Al 0.01 ) O 30 |
| synthetic | K | □ Well | Mg 2 | Mg Zn 2 | Si 12 O 30 | |
| synthetic | Rb | □ Well | Mg 2 | Mg Zn 2 | Si 12 O 30 | |
| synthetic | N / A | □ Well | (Mg, Co) 2 | (Mg, Co) 3 | Si 12 O 30 | |
| synthetic | K | □ Well | (Mg, Co) 2 | (Mg, Co) 3 | Si 12 O 30 | |
| synthetic | K | □ K | (Mg, Co) 2 | (Mg, Co) 3 | Si 12 O 30 | |
| synthetic | Rb | □ Well | (Mg, Co) 2 | (Mg, Co) 3 | Si 12 O 30 | |
| Friedrichbeckeit | K | □ Well | Mg 2 | Be 3 | Si 12 O 30 | K 0.87 Na 0.86 (Mg 1.57 Mn 0.28 Fe 0.24 ) Σ2.09 (Be 1.83 Mg 1.17 ) Σ3.00 [Si 12 O 30 ] |
| Klochit | K | □ 2 | Fe 2+ Fe 3+ | Zn 3 | Si 12 O 30 | (K 0.78 Na 0.22 ) (□ 1.67 Na 0.33 ) (Fe 3+ 1.19 Fe 2+ 0.45 Mn 0.27 Ca 0.04 Co 0.02 Ni 0.01 Mg 0.01 Ti 0.01 ) (□ 0.25 Zn 2.63 Fe 2+ 0.10 Li 0.02 ) [Si 12 O 30 ] |
| Agakhanovite- (Y) | K | □ H 2 O | Ca Y | Be 3 | Si 12 O 30 | K 1.00 (H 2 O) 0.92 Na 0.02 (Y 0.89 Yb 0.01 Ca 1.06 ) А1.96 (Be 2.93 Al 0.07 ) А3.00 Si 12.02 O 30 |
| Agakhanovite (Ce) | K | □ H 2 O | Ca Ce | Be 3 | Si 12 O 30 | hypothetical end link |
| Oftedalit | K | □ 2 | Ca Sc | Be 3 | Si 12 O 30 | K 0.98 (Sc 0.96 Ca 0.79 Mn 2+ 0.18 Fe 2+ 0.04 ) (Be 2.91 Al 0.09 ) Si 11.98 O 30 |
| 2M 2+ M 3+ on T2 | ||||||
| Milarite | K | □ H 2 O | Approx 2 | Be 2 Al | Si 12 O 30 | K 2.09 Na 0.3 Ca 4.17 Be 4.19 Al 1.83 Si 23.85 O 60 |
| Laurent thomasite | K | □ 2 | Mg 2 | Be 2 Al | Si 12 O 30 | |
| Almarudit | K | □ 2 | (Mn, Fe, Mg) 2 | Be 2 Al | Si 12 O 30 | K 0.86 Na 0.21 (Mn 1.03 Fe 0.62 Mg 0.38 Ca 0.02 ) (Be 2.07 Al 0.80 Zn 0.03 ) Si 12.05 O 30 |
| Yagiit | N / A | □ 2 | Mg 2 | Mg 2 Al | Si 12 O 30 | (Na 0.70 K 0.30 ) (Na 0.50 ) (Mg 2.00 ) (Mg 0.60 Fe 0.34 Ti 0.10 Al 1.96 ) (Si 10.22 Al 1.78 ) O 30 |
| Chayesite | K | □ 2 | Mg 2 | Mg 2 Fe 3+ | Si 12 O 30 | K (K 0.14 Na 0.10 ) (Mg 3.29 Fe 2+ 0.67 Mn 0.04 ) (Fe 3+ 0.64 Fe 2+ 0.29 Al 0.04 Ti 0.03 ) Si 12 O 30 |
| Trattnerite | □ | □ 2 | Fe 3+ Mg | Mg 2 Fe 3+ | Si 12 O 30 | C (Na 0.01 K 0.07 ) A, T2 (Fe 3+ 1.99 Ti 0.01 Mg 2.46 Fe 2+ 0.30 Mn 2+ 0.08 Zn 0.05 Al 0.04 ) T1 [Si 12 O 30 ] |
| 3M 3+ on T2 | ||||||
| Poudretteit | K | □ 2 | Well 2 | B 3 | Si 12 O 30 | K 1.00 (Na 1.87 K 0.04 ) B 3.05 Si 12.14 O 30 |
| synthetic alkali-free end link | □ | □ 2 | Mg 2 | Al 3 | AlSi 11 O 30 | □ □ 2 (Mg 1.65 Al 0.35 ) Al 3 [Si 10.4 Al 1.6 ] O 30 |
| Osumilith | K | □ 2 | Fe 2+ 2 | Al 3 | Al 2 Si 10 O 30 | (K 0.65 Na 0.33 Ca 0.02 ) (Fe 2+ 0.96 Mg 0.93 Mn 0.11 ) (Fe 3+ 0.24 Al 2.63 ) (Al 1.62 Si 10, 34 O 30 ) |
| Osumilite (Mg) | K | □ 2 | Mg 2 | Al 3 | Al 2 Si 10 O 30 | (K 0.72 Na 0.03 Ca 0.01 ) (Mg 1.97 Mn 0.04 ) (Al 2.53 Fe 3+ 0.45 ) [Si 10.32 Al 1.68 ] O 30 |
| Armenite | Ba | 2H 2 O | Approx 2 | Al 3 | Al 3 Si 9 O 30 | Ba 0.991 K 0.029 Na 0.082 Ca 2.016 Al 5.864 Si 9.054 O 30 * 2H 2 O |
| synthetic Ba-Osumilite | Ba | □ 2 | Mg 2 | Al 3 | Al 3 Si 9 O 30 | |
| synthetic Sr-Osumilite | Sr | □ 2 | Mg 2 | Al 3 | Al 3 Si 9 O 30 | |
| hypothetical alkali-free end link | □ | □ 2 | Al 2 | Al 3 | Al 3 Si 9 O 30 | □ □ 2 Al 2 Al 3 [Si 9 Al 3 ] O 30 |
Simple substitutions
In the milarite group, mixed crystals are common , which result from the exchange of cations of the same charge on a lattice position. In this way, Mg, Fe 2+ and Mn 2+ can replace each other in the A and T2 positions . Osumilite-Osumilite (Mg) mixed crystals or compositions between Roedderite, Merrihueit and Klöchite are based on this.
The interchangeability of Zr 4+ , Ti 4+ and Sn 4+ in the A position leads to Berezanskite, Brannockite, Sogdianite mixed crystals.
In addition, some rows of mixed crystals were described in which the compositions were described by the coupled exchange of differently charged cations at different positions.
Coupled substitutions on the [6] A and [9] B positions
B □ + A Zr 4+ = B Na + A Fe 3+ : Sogdianite-sugilite mixed crystals across the entire composition range are known from the debris of the Darai-Pioz glacier.
B □ + A Mg = B Na + A Na: This exchange reaction describes Roedderite-Eifelite mixed crystals from the contact metamorphic xenolites in the leucite - tephrite - lavas of the Bellerberg volcano in the Eifel , Germany.
Coupled substitution on the [4] T2 and [9] B positions
B Na + T2 Mg 2+ = B □ + T2 Fe 3+ : This exchange reaction forms the basis of the Roedderite-Chayesite mixed crystals, which were described as an example from the lamproites near Cancarix, Albacete , Spain .
B Na + T2 Be = B □ + T2 Al: This reaction contributes significantly to the increase in the Be content of milarite to values above 2 apfu.
Coupled substitutions at the [4] T2 and [6] A positions
A Ca + T2 Al = A Y + T2 Be: This reaction describes the milarite-agakhanovite-Y mixed crystal series. It leads to an increase in the Be content of milarite and is the main mechanism by which rare earth elements such as yttrium (Y) can be incorporated into the milarite structure.
Coupled substitutions with cations at the [12] C position
C K + + T1 Al 3+ = C □ + T1 Si 4+ : This is one of the reactions via which the occupation of the 12-fold coordinated C position can be reduced. It leads to K contents below 1 apfu and Si contents above 10 apfu in Osumilithen.
[C] K + + [A] Mg 2+ = [C] □ + [A] Al 3+ : Another reaction through which the occupation of the 12-coordinate C position can be reduced. It also leads to K contents below 1 apfu and Al contents above 5 apfu in Osumilithen.
use
Natural minerals of the milarite group are not used for technical purposes. Only poudretteite is cut into gemstones and is an expensive rarity.
Synthetic Ba-Osumilite is the main component of glass ceramics , which can withstand very high temperatures (around 1300 ° C). Ba-Osumilith glass ceramics have a low coefficient of thermal expansion , do not react with silicon carbide (SiC) and can therefore be reinforced with SiC fibers. Such high-temperature-resistant materials are of interest. B. for components in jet engines. Because of their very favorable dielectric properties (very low dielectric constant ), radar and microwave radiation are hardly absorbed. Ba-Osumilith ceramics are therefore well suited for antenna domes z. B. on aircraft.
Individual evidence
- ↑ a b c d e f g h i j k l FC Hawthorne, M. Kimata, P. Černý, N. Ball, GR Rossman, JD Grice: The crystal chemistry of the milarite-group minerals. In: American Mineralogist. Volume 76, 1991, pp. 1836-1856 ( PDF, 2.6MB )
- ↑ a b c d e f g P. Cerny, FC Hawthorne, E. Jarosewich: Crystal Chemistry of Milarite. In: Canadian Mineralogist. Volume 18, 1980, pp. 41–57 ( PDF, 833 kB )
- ↑ a b A. Kenngott: Communications to Professor G. Leonhard. In: New Yearbook for Mineralogy, Geology and Paleontology. 1890, 1870, pp. 80–81 ( PDF, 250 kB )
- ^ C. Palache: On the presence of beryllium in milarite. In: American Mineralogist. 16, 1931, pp. 469–470 ( PDF, 111 kB )
- ↑ H. Neumann: Armenite, a new mineral. In: Norsk Geologisk Tidsskrift. 19, 1939, pp. 312–313 ( PDF, 55 kB )
- ^ A b H. Neumann: Armenite, a water-bearing barium-calcium-alumosilicate. In: Norsk Geologisk Tidsskrift. 21, 1941, pp. 19–24 ( PDF, 683 kB )
- ↑ NV Belov, TN Tarkova: Crystal structure of milarite. In: Dokl. Akad. Nauk SSSR 69 1949, pp. 365-368.
- ↑ NV Belov, TN Tarkova: Crystal structure of milarite. In: Trudy. lnst. Krist., A kad. Nauk SSSR 6 1951, pp. 83-140.
- ^ A. Miyashiro: Osumilite, a New Mineral, and Cordierite in Volcanic Rocks. In: Proceedings of the Japan Academy. 29, 1953, pp. 321–323 ( PDF, 109 kB )
- ^ A. Miyashiro: Osumilite, a new silicate mineral, and its crystal structure. In: American Mineralogist. 41, 1956, pp. 104–116 ( PDF, 736 kB )
- ^ A b RT Dodd (Jr.), W. Randall van Schmus, Ursula B. Marvin: Merrihueite, A New Alkali-Ferromagnesian Silicate from the Mezo-Madaras Chondrite. In: Science. 149, 1965, pp. 972–974 ( PDF, 844 kB )
- ^ A b LH Fuchs, C. Frondel, C. Klein (Jr.): Roedderite, A New Mineral From The Indarch Meteorite. In: American Mineralogist. 51, 1966, pp. 949–955 ( PDF, 447 kB )
- ↑ a b TE Bunch, LH Fuchs: Yagiite, A New Sodium-Magnesium Analogue Of Osumilite. In: American Mineralogist. 54, 1969, pp. 14-18. ( PDF, 321 kB )
- ^ HA Ishii, JP Bradley, ZR Dai, M. Chi, AT Kearsley, MJ Burchell, ND Browning, F. Molster: Comparison of comet 81P / Wild 2 dust with interplanetary dust from comets. In: Science. Volume 319 (5862), 2008, pp. 447-450. ( PDF 423.5 kB )
- ↑ T. Noguchi, N. Ohashi, S. Tsujimoto, T. Mitsunari, JP Bradley, T. Nakamura, S. Toh, T. Stephan, N. Iwata: Cometary dust in Antarctic ice and snow: Past and present chondritic porous micrometeorites preserved on the Earth's surface. In: Earth and Planetary Science Letters. Volume 410, 2015, pp. 1-11. ( Abstract )
- ↑ Darai-Pioz Glacier (Dara-i-Pioz; Dara-Pioz), Alai Range (Alayskiy), Tien Shan Mtn, Region of Republican Subordination, Tajikistan
- ^ VD Dusmatov, AF Efimov, ZT Kataeva, LA Khoroshilova, KP Yanulov: Sogdianite - a new mineral. In: Doklady Akademii Nauk SSSR. 182, 1968, pp. 1176-1177. ( PDF (Russian) )
- ↑ M. Fleischer: New mineral names. In: American Mineralogist. 54, 1969, pp. 1218-1223. ( PDF, 388 kB )
- ↑ E. l. Semenov, VD Dusmatov, AP Khomyakov, AA Voronkov, ME Kasakova: Darapiosite, a new mineral of the milarite group. In: Zapiski Vses. Mineralogist Obshch. 104, 1975, pp. 583-585. (in Russian)
- ↑ M. Fleischer: New mineral names. In: American Mineralogist. 61, 1976, pp. 1053-1054. ( PDF, 388 kB )
- ^ A b EV Sokolova, LA Pautov, VA Zharikov: Crystal structure of Dusmatovite. In: Doklady Physics. 40, 1995, pp. 53-506. ( PDF, 514 kB )
- ^ Dusmatovite
- ↑ LA Pautov, AA Agakhonov: Berezanskite, K Li3 Ti2 Si12 O30, a new mineral. In: Zapiski Vseross. Mineral. Obshch. 126 (4), 1997, pp. 75-80. ( PDF (English summary), 95 kB )
- ↑ a b J. Jambor et al.: New Mineral Names - Berezanskite. Abstract in: American Mineralogist. 83, 1998, p. 907. ( PDF, 71 kB )
- ↑ LA Pautov, AA Agakhanov, EV Sokolova: Shibkovite K (Ca, Mn, Na) 2 (K2-x □ x) 2Zn3Si12O30 - the new mineral from the milarite group. In: Zapiski Vserossijskogo Mineralogicheskogo Obshchestva. 127 (4), 1998, pp. 89-94. ( PDF, 541 kB )
- ↑ a b J. Jambor et al.: New Mineral Names - Shibkovite. Abstract in: American Mineralogist , 85, 2000, p. 628. ( PDF, 34 kB )
- ↑ a b FC Hawthorne, YA Abdu, NA Ball, P. Černý, R. Kristiansen: Agakhanovite- (Y), ideally (YCa) □ 2KBe3Si12O30, a new milarite-group mineral from the Heftetjern pegmatite, Tørdal, Southern Norway: Description and crystal structure. In: American Mineralogist. 99, 2014, pp. 2084–2088 ( PDF, abstract )
- ↑ a b MA Cooper, FC Hawthorne, NABall, P. Cerny, R. Kristiansen: Oftedalite, (Sc, Ca, Mn2 +) 2 K (Be, Al) 3 Si12 O30, a new member of the milarite group from the Heftetjern pegmatite , Tørdal, Norway: description and crystal structure. In: Canadian Mineralogist. 44, 2006, pp. 943-949. ( PDF, 347 kB )
- ↑ T. Armbruster, M. Czank: H20 ordering and superstructures in armenite, BaCa2AlSi9030.2H20: A sinlgle-crystal X-ray and TEM study. In: American Mineralogist. Volume 77, 1992, pp. 422-430 ( PDF, 2.0MB )
- ↑ a b c d T. Armbruster, R. Oberhänsli: Crystal chemistry of double-ring silicates: Structures of sugilite and brannockite. In: American Mineralogist. 73, 1988, pp. 595-600. ( PDF, 1.1 MB )
- ↑ CA Geiger: A 57 Fe Mössbauer Spectroscopic Study Of Sugilite, KNa2 (Fe3 +, Mn3 +, Al) 2 Li3 Si12 O30. In: The Canadian Mineralogist. 47, 2009, pp. 927-931. ( PDF, 659 kB )
- ↑ M. Nagashima, C. Fukuda, T. Matsumoto, T. Imaoka, G. Odicino and G. Armellino: Aluminosugilite, IMA 2018-142. CNMNC Newsletter No. 49 . In: European Journal of Mineralogy . tape 31 , 2019, pp. 653–658 ( schweizerbart.de [PDF; 320 kB ; accessed on September 8, 2019]).
- ↑ a b c d e f g h i FC Hawthorne: The Use Of End-Member Charge-Arrangements In Defining New Mineral Species And Heterovalent Substitutions In Complex Minerals. In: The Canadian Mineralogist. 40, 2002, pp. 699-710. ( PDF (309 kB) )
- ↑ a b c d e f g A. Wohlfart, W. Eysel: Crystal Chemical Investigations Of Compounds With Milarite Structure . In: GEO Berlin '98 . October 1998, p. P209-P210 .
- Jump up ↑ JS White Jr., JE Arem, JA Nelen, PB Leavens, RW Thomssen: Brannockite, A New Tin Mineral. In: The Mineralogical Record. 4, 1973, pp. 73-76. ( PDF 2.1 MB )
- ^ A b EV Sokolova, FC Hawthorne, LA Pautov: The Crystal Chemistry Of Li-bearing Minerals With The Milarite-Type Structure: The Crystal Structure Of End-Member Sogdianite. In: The Canadian Mineralogist. 38, 2000, pp. 853-859. ( PDF, 698 kB )
- ^ MA Cooper, FC Hawthorne, ES Grew: The crystal chemistry of sogdianite, a milarite-group mineral. In: American Mineralogist. 84, 1999, pp. 764-768. ( PDF, 1.4 MB )
- ↑ G. Ferraris, M. Prencipe, LA Pautov, EV Sokolova: The Crystal Structure Of Darapiosite And A Comparison With Li And Zn-Bearing Minerals Of The Milarite Group. In: The Canadian Mineralogist. 37, 1999, pp. 769-774. ( PDF, 1.4 MB )
- ↑ a b c d e f N. Nguyen, J. Choisnet, B. Raveau: Silicates synthetiques a structure milarite . In: Journal of Solid State Chemistry . tape 34 , August 1980, p. 1-9 ( abstract ).
- ↑ a b K. Abraham, W. Gebert, O. Medenbach, W. Schreyer, G. Hentschel: Eifelite, KNa3Mg4Si12O30, a new mineral of the osumilite group with octahedral sodium. In: Contributions to Mineralogy and Petrology. 82, 1983, pp. 252-258. ( doi: 10.1007 / BF01166619 )
- ↑ TN Nadezhina, EV Sokolova, DI Belakovskii: Crystal structure of K (K0.58 Na0.42) 2 Zn3 Mn1.5 [Si12 O30], a new natural representative of the milarite structural type . In: Dokl. Akad. Nauk SSSR . tape 313 , August 1990, p. 865-868 ( PDF ).
- ^ G. Artioli, I. Angelini, F. Nestola: New milarite / osumilite-type phase formed during ancient glazing of an Egyptian scarab. In: Applied Physics A. Volume 110, 2013, pp. 371-377. ( doi: 10.1007 / s00339-012-7125-x )
- ↑ PA Sandomirskii, MA Simonov, NV Belov: Crystal structure of synthetic Mn-milarite K2Mn5 [Si12O30] · H2O . In: Soviet Physics Doklady . tape February 22 , 1977, p. 181 , bibcode : 1977SPhD ... 22..181S .
- ↑ C. Lengauer, N. Hrauda, U. Kolitsch, R. Krickl, E. Tillmanns: Friedrichbeckeite, K (□ 0.5Na0.5) 2 (Mg0.8Mn0.1Fe0.1) 2 (Be0.6 Mg0.4) 3 [Si12O30], a new milarite-type mineral from the Bellerberg volcano, Eifel area, Germany. In: Mineralogy and Petrology. 96, 2009, pp. 221-232. ( Abstract )
- ↑ H.-P. Bojar, F. Walter, C. Hauzenberger, W. Postl: Klöchite, K □ 2 (Fe2 + Fe3 +) Zn3 [Si12O30], A New Milarite-Type Mineral Species From The Klöch Volcano, Styria, Austria. In: Canadian Mineralogist. 49, 2011, pp. 1115-1124. ( Abstract )
- ↑ M. Novák, J. Cícha, R. Čopjaková, R. Škoda, M. Vašinová Galiová: Primary (magmatic?) And hydrothermal milarite-group minerals from the Velká skála pegmatite, Písek pegmatite district, Czech Republic . In: Second Eugene E. Foord Pegmatite Symposium, July 15-19, 2016 Colorado School of Mines campus, Golden, Colorado . July 2016, p. 70-72 ( PDF (17.2 MB) ).
- ↑ C. Ferraris, I. Pignatelli, F. Cámara, S. Ponti, M. Schreyer, GC Parodi, and F. Wei: Laurentthomasite, IMA 2018-157. CNMNC Newsletter No. 49 . In: European Journal of Mineralogy . tape 31 , 2019, pp. 653–658 ( schweizerbart.de [PDF; 320 kB ; accessed on September 8, 2019]).
- ↑ T. Mihajlovic, CL Lengauer, T. Ntaflos, U. Kolitsch, E. Tillmanns: Two new minerals, rondorfite, Ca8Mg [SiO4] 4Cl2, and almarudite, K (□, Na) 2 (Mn, Fe, Mg) 2 (Be, Al) 3 [Si12O30], and a study of iron-rich wadalite, Ca12 [(Al8Si4Fe2) O32] C16, from the Bellerberg (Bellberg) volcano, Eifel, Germany. In: New Yearbook for Mineralogy, Treatises. 149, 2004, pp. 265-294. ( Summary PDF 250 kB )
- ↑ CNMMN / CNMNC, IMA: The New IMA List of Minerals - A Work in Progress - Updated: September 2016 . September 2016 ( PDF ).
- ↑ D. Velde, O. Medenbach, C. Wagner, W. Schreyer: Chayesite, K (Mg, Fe2 +) 4Fe3 + [Si12O30]: A new rock-forming silicate mineral of the osumilite group from the Moon Canyon (Utah) lamproite. In: American Mineralogist. 74, 1989, pp. 1368-1373. ( PDF 489 kB )
- ^ W. Postl, F. Walter, K. Ettinger, C. Hauzenberger, HP Bojar: Trattnerite, (Fe, Mg) 2 (Mg, Fe) 3 [Si12 O30], a new mineral of the milarite group: mineral data and crystal structure. In: Eur. J. Mineral. 16, 2004, pp. 375-380. ( PDF, 450 kB )
- ↑ JD Grice, TS Ercit, J. van Velthuizen, PJ Dunn: Poudretteite, KNaB3Si12O3, A New Member Of The Osumilite Group From Mont Saint-Hilaire, Ouebec, And Its Crystal Structure. In: Canadian Mineralogist. 25, 1987, pp. 763-766. ( PDF, 556 kB )
- ↑ a b c d T. Armbruster, R. Oberhänsli: Crystal chemistry of double-ring silicates: Structural, chemical, and optical variation in osumilites. In: American Mineralogist. Volume 73, 1988, pp. 585-594. ( PDF (1.3 MB) )
- ↑ a b c W. Winter, T. Armbruster, C. Lengauer: Crystal structure refinement of synthetic osumilite-type phases: BaMg2A16Si9O30, SrMg2AI6Si9O30 and Mg2Al4Si11O30 . In: European Journal Of Mineralogie . tape 7 , 1995, p. 277-286 ( abstract ).
- ↑ E. Olsen, TE Bunch: Compositions Of Natural Osumilites . In: The American Mineralogist . tape 55 , May 1970, pp. 875-879 ( PDF, 328 kB ).
- ^ NV Chukanov, I. Pekov, RK Rastsvetaeva, SM Aksenov, D. Belakovskiy, V. Van Kim: Osumilite- (Mg): Validation as a Mineral Species and New Data. In: Geology of Ore Deposits. 55, 2013, pp. 265-294. ( doi: 10.1134 / S1075701513070064 )
- ↑ a b GH Beall, AM Chirino, K. Chyung, FW Martin, MP Taylor: Glass-ceramic articles containing osumilite . August 1984 (English, full text ).
- ↑ LA Pautov, PV Khvorov, VA Muftakhov, AA Agakhanov: Sogdianite and sugilite from Dara-i-Pioz massif (Tajikistan). In: Proceedings of the Russian Mineralogical Society. Volume 129, 2000, pp. 66-79. ( Abstract, PDF 556 kB )
- ↑ E. Alietti, MF Brigatti, p Capredi, L. Poppi: The roedderite-chayesite series from Spanish lamproites: crystal-chemical characterization. In: Mineralogical Magazine. Volume 58, 1994, pp. 655-662. ( PDF 556 kB )